May 29, 2015 report
Mechanism for aprotic sodium-air batteries
The automobile industry has been interested in finding batteries that allow electric cars to travel at a comparable distance to gas-powered cars. Currently, electric cars use a lithium ion battery, but there is interest in a metal battery that uses air as the cathode because the battery would not need to house the cathode solution. Originally, researchers focused on lithium-air batteries because energy density is similar to gasoline. However, several technical barriers have stymied progress with lithium-air batteries.
Now researchers are turning to sodium-air batteries, which have a lower energy density, but are still better than lithium-ion batteries in many respects. Sodium-air batteries do not have some of the technical issues that lithium-air batteries do, giving the sodium-air batteries a longer lifespan. As part of an effort to understand the difference between lithium- and sodium-air batteries, Chun Xia, Robert Black, Russel Fernandes, Brian Adams, and Linda F. Nazar of the University of Waterloo discovered the role of a phase transfer catalysts in the sodium-air battery reaction mechanism. Their studies were recently published in Nature Chemistry.
In sodium-air batteries, the sodium is the anode and the cathode is a porous carbon that traps molecular oxygen. The battery undergoes two stages, charge and discharge. When the battery is discharged (i.e., no potential is added to the system), the sodium is oxidized, forming sodium ion (Na+). The sodium cation travels through the electrolyte and meets up with the accompanying electron passed through the external circuit that reduces the adsorbed O2. This forms NaO2, which can be reversibly reduced back to Na+ and O2.
In lithium batteries, LiO2 forms Li2O2 along with carbonate decomposition products when LiO2 reacts with the organic electrolyte. These by-products increase the charging voltage of the lithium-air battery because they cannot be easily reversibly oxidized. The voltage for charging Li2O2 is also high, which exacerbates electrolyte decomposition. Sodium batteries, on the other hand, form less of these by-products and do not undergo subsequent reactions to form Na2O2. Prior studies have shown that NaO2 is stable at the nanoscale, although Na2O2 is more stable at the bulk level. The current study shows why NaO2 is the preferred product in the sodium-air battery and why the cell can be charged so much more easily than a lithium-air cell.
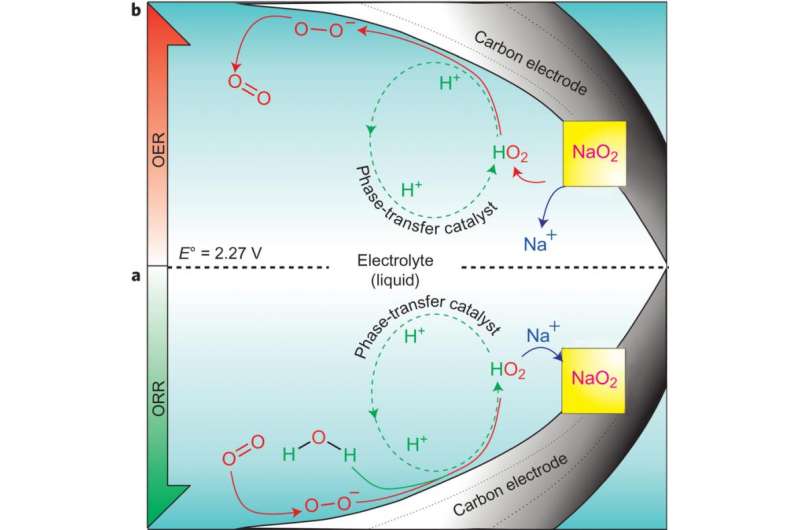
Researchers know that after discharge in a sodium-air battery, NaO2 crystals form on cathode. Xia, et al. demonstrate that this process is almost exclusively driven by the presence of a proton phase-transfer catalyst (PPTC). The effects of the PPTC were tested by comparing the sodium-air battery capacity and NaO2 crystal formation in pure anhydrous sodium triflate and in sodium triflate with the addition of water as a source of protons. Their results showed that when a trace amount of water was added to the electrolyte, the discharge capacity of the battery greatly increased compared to when there was no water present, and NaO2 crystals formed. This effect increased as more water was added up to 100ppm until the effect started declining, indicating catalytic behavior.
This study also showed that oxygen evolution was almost zero in the anhydrous environment but remained constant when water was added to the system, the PPTC is vital to oxygen evolution on charge, and hence reversibility..
Xia, et al. show that the mechanism at work in the sodium-air battery relies on the PPTC. After discharge, oxygen is reduced to a superoxide which reacts with H2O to yield HO2 and OH-. HO2, which is a soluble intermediate, desorbs from the electrode surface and reacts with Na+ to form nuclei of NaO2, prompting crystal growth. The formation of NaO2 from HO2 causes the catalyst, H+, to be released to then react with more superoxide on the cathode surface. This mechanism was confirmed using rotating disk electrode studies.
More importantly, Xia, et al. tested the reverse reaction, or the battery charging, as further verification that H+ is functioning as a catalyst. In the reverse reaction, H+ should react with NaO2 crystals to form HO2 which would then deposit superoxide on the cathode surface. They found that while the reverse reaction required prohibitively high charge potentials in an anhydrous electrolyte, the reverse reaction occurred readily in the presence of water. This study demonstrates that the equivalent catalyzed reactions cannot occur in the Lithium-air cell because the forward and reverse directions follow a different pathway.
Areas of further research would address the reactivity of HO2, which can undergo side reactions with an organic electrolyte. Also, the formation of NaO2 crystals may form at locations other than cathode. However, both of these factors may be overcome with further improvements to the sodium-air cell.
More information: "The critical role of phase-transfer catalysis in aprotic sodium oxygen batteries" Nature Chemistry, DOI: 10.1038/nchem.2260
Abstract
In the search for improved energy storage, rechargeable metal–oxygen batteries are very attractive owing to their reliance on molecular oxygen, which forms oxides on discharge that decompose reversibly on charge. Much focus has been directed at aprotic Li–O2 cells, but the aprotic Na–O2 system is of equal interest because of its better reversibility. We report here on the critical role and mechanism of phase-transfer catalysis in Na–O2 batteries. We find that it is solely responsible for the growth and dissolution of micrometre-sized cubic NaO2 crystals and for the reversible cell capacity. In the absence of phase-transfer catalysis, quasi-amorphous NaO2 films are formed and cells exhibit negligible capacity. Electrochemical investigations provide a measure of the transportation of superoxide from the carbon electrode to the electrolyte phase by the phase transfer catalyst. This leads to a new understanding of the mechanism of Na–O2 batteries that, significantly, extends to Li–O2 cells and explains their different behaviour.
© 2015 TechXplore