July 22, 2015 weblog
A general model for optimizing the electrolyte used in lithium-air batteries
Metal-air batteries are attractive prospects for creating a battery that is light-weight, high-powered, and long-lasting. However, one of the problems keeping lithium-air batteries from practical use is the accumulation of solid material on the carbon cathode.
A collaboration of researchers hailing from the University of California at Berkeley, Lawrence Berkeley National Laboratory, Carnegie Mellon University, and the Institute for Combustion Technology in Germany have demonstrated that an electrolyte comprised of anions with a high donor number and a non-aqueous solvent with a lower donor number increases a lithium-air battery's capacity. Their work is published in The Proceedings of the National Academy of Sciences.
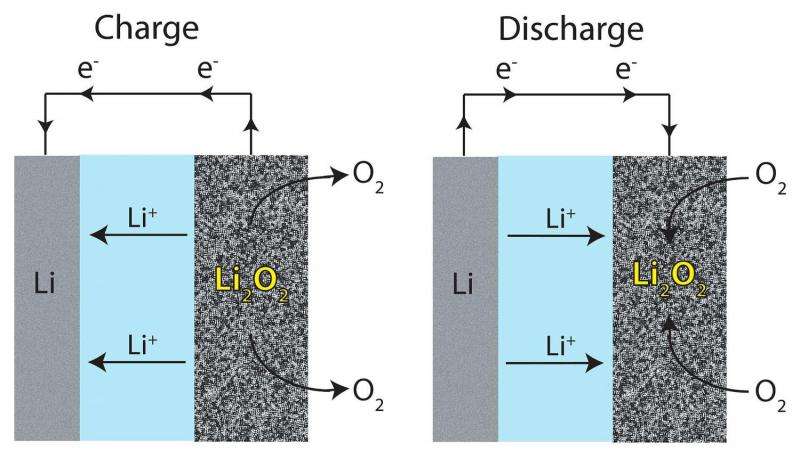
In non-aqueous lithium-air (Li-O2) batteries, the product from the electrochemical reaction is lithium peroxide (Li2O2). Lithium peroxide is insoluble in aprotic, organic electrolytes. It deposits on the cathode surface, which eventually insulates the cathode from further reaction and diminishes the cell's capacity.
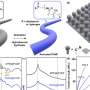
One way that several groups have tried to overcome this problem is to tailor the electrolyte to enhance the solubility of intermediate products. In this study, Burke et al. conducted both qualitative and quantitative studies testing how a LiNO3/LiTFSI/DME electrolyte enhances the cell's capacity by solvating LiO2, an intermediate in the electrochemical reaction. This allows for more Li+ and O2- in solution, increasing the capacity of the cell.
To design their electrolytic solution, Burke et al. decided to work with two salts with two different donor numbers. One donor number (NO3-) was higher than the solvent, 1,2-dimethoxyethane (DME), while the other (TSFI-) was lower. Gutmann donor numbers are a measure of the Lewis basicity of a solvent. In other words, it is a measure of how well the salt or solvent attracts positively charged species in solution.
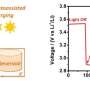
The first step was to see how the anion affected discharge performance. Burke et al. tested Li-O2 batteries that had various concentrations of LiNO3 and LiTFSI salts. The concentration of Li+ was always 1.0 M in DME. In their study, cell capacity increased more than four-fold as the concentration of LiNO3 increased, confirming that anion selection plays a role in the cell cycle process.
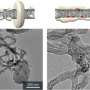
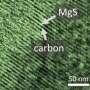
They then used scanning electron microscopy to examine the morphology of Li2O2 deposits on the cathode surface. Deposition morphology can be used to determine how Li2O2 formed. Li2O2 morphology indicated that it was formed from LiO2 in solution. Therefore, the presence of NO3- must have made the intermediate more soluble. Cell capacity likely increased due to the presence of NO3- which induced soluble O2-.
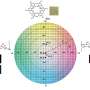
7Li NMR was used to investigate the electron donicity felt by Li+ ions. Prior research had shown that NMR can be used as a qualitative tool to determine the relative effect of the anion on electrolyte donor number by looking at shifts in Li+ peaks. These shifts correspond to shielding due to electrolyte composition. NMR studies showed that as LiNO3 concentration increased, the 7Li peak shifted downfield (i.e., less shielded) further confirming that NO3- plays a role in the electrolyte's donicity.
Burke et al. also investigated the thermodynamic properties of the solution to provide a quantitative model for how anion donor number affects cell capacity. They began by using a model often used to describe ferromagnetism (Ising Model) to describe the Li+ solvation shell. This model accounts for the various interactions between neighboring particles. They then simplified this model by using mean-field approximation.
Burke et al. mathematically show that the energetics of Li+ solvation is a function of the donor number of the solvent. If we think of the Li+ solvation shell as having a fixed number of locations that can be occupied by one of the electrolyte species, then what occurred in this study was that NO3-, which has a higher donor number, displaced DME in some of the locations. As concentration of NO3- increased, it occupied more locations within the solvation shell.
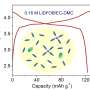
From this model, Burke at al. created a contour map that provides a generalized tool for relating the free energy of Li+ to donor number of the solvent and salt anion. In general, anions with a higher donor number than the solvent stabilize Li+ so insoluble LiO2 does not form, resulting in a higher cell capacity.
This work shows that a tailor-made electrolyte solution that targets intermediate species, specifically the solvation properties of the species, is a way to overcome one of the limitations of Li-O2 batteries and enhance cell capacity. Furthermore, their model may be broadly applicable in other metal-gas cells.
More information: "Enhancing electrochemical intermediate solvation through electrolyte anion selection to increase nonaqueous Li-O2 battery capacity", PNAS , DOI: 10.1073/pnas.1505728112
© 2015 Tech Xplore