October 9, 2015 feature
Scientists convert harmful algal blooms into high-performance battery electrodes

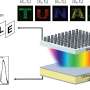
Last August, the seasonal harmful algal blooms (HABs) in Lake Erie grew so extreme that they poisoned the water system in Toledo, Ohio, leaving nearly half a million residents without drinking water. But a few researchers at the time collected some of the toxic HABs, and have now shown that, by heating them at temperatures of 700-1000 °C in argon gas, the HABs can be converted into a material called "hard carbon" that can be used as high-capacity, low-cost electrodes for sodium-ion (Na-ion) batteries.
The researchers, led by environmental engineer Dr. Da Deng at Wayne State University in Detroit, have published a paper on using HABs for electrochemical energy storage in a recent issue of Environmental Science & Technology.
"Harmful algal blooms, caused by cyanobacteria (or so called 'blue-green algae'), severely threaten humans, livestock, and wildlife, leading to illness and sometimes even death," Deng told Phys.org. "The Toledo water crisis in 2014 caused by HABs in Lake Erie is a vivid example of their powerful and destructive impact. The existing technologies to mitigate HABs are considered a 'passive' technology and have certain limitations. It would significantly and broadly impact our society and environment if alternative technologies could be developed to convert the HABs into functional high-value products."
As Deng explained, one such high-value product may be electrodes for Na-ion batteries, which have the potential to replace the dominantly used lithium-ion (Li-ion) batteries.
"We demonstrated the conversion of HABs, freshly collected from Lake Erie near Toledo, into high-performance electrodes for Na-ion batteries," he said. "We call it a 'trash-to-treasure' approach. This technology could be promising for mitigating HABs to overcome their environmental threats and providing 'green' electrodes for reversible sodium storage in Na-ion batteries."
As Deng explained, Na-ion battery technology is still in its infancy compared to Li-ion batteries. One of the challenges in developing Na-ion batteries is to find a reliable electrode material. While graphite is often used in the electrodes of Li-ion batteries, the larger Na ions do not fit as well into the graphite structure as the smaller Li ions do. Instead, Na ions fit better into hard carbon, which is more disordered than graphite and contains a greater number of large defects and voids that can store the larger Na ions.

While hard carbon is most often derived from petroleum, it can also be made from biomass. This study is the first time that HABs (specifically blue-green algae) have been directly converted into carbon for Na-ion batteries. HABs have advantages in that they grow quickly and don't require land or soil. And as the researchers showed here, HABs can easily be converted into hard carbon by simple heat treatment, without the need for purification or other additional processes.
After heating the algae, the researchers made the electrodes out of a mixture of 80% hard carbon derived from algae, 10% carbon black (to enhance conductivity) and 10% binder. After drying this slurry overnight, they assembled it into coin cells with sodium foil as the counter electrode. Tests showed that the electrodes start out with a high capacity of up to 440 mAh/g, but suffer from an irreversible capacity loss after the first cycle, bringing the capacity down to about 230 mAh/g. The electrodes then have good capacity retention from the second cycle onward. The researchers also found that some performance factors, including capacity and stability, depend on the temperature at which the algae was heated, which points to a way to improve their performance in the future.
"Our future research will focus on the optimization of electrochemical performance of HAB-derived carbon in Na-ion batteries," Deng said. "We will try to address the issue of first-cycle irreversible capacity loss in Na-ion batteries. We are also interested in developing methods for the large-scale harvesting of HABs and studying their implications on the ecosystem."
Overall, the researchers explain that this "trash to treasure" conversion process addresses two problems at once: mitigating the HAB problem in freshwater lakes, and providing a useful electrode material for Na-ion batteries, which have a large potential cost advantage over Li-ion batteries. To illustrate, the researchers note that Li-ion batteries cost about $410 per kilowatt-hour (kWh) in 2014, whereas the average retail price of electricity in the US is about 10 cents per kWh. Because sodium is much more abundant than lithium, Na-ion batteries are expected to greatly decrease this cost in the future.
More information: Da Deng, et al. "Trash to Treasure: From Harmful Algal Blooms to High-Performance Electrodes for Sodium-Ion Batteries." Environmental Science & Technology. DOI: 10.1021/acs.est.5b03882
© 2015 Phys.org