Chemical cluster could transform energy storage for large electrical grids
To power entire communities with clean energy, such as solar and wind power, a reliable backup storage system is needed to provide energy when the wind isn't blowing and the sun isn't out.
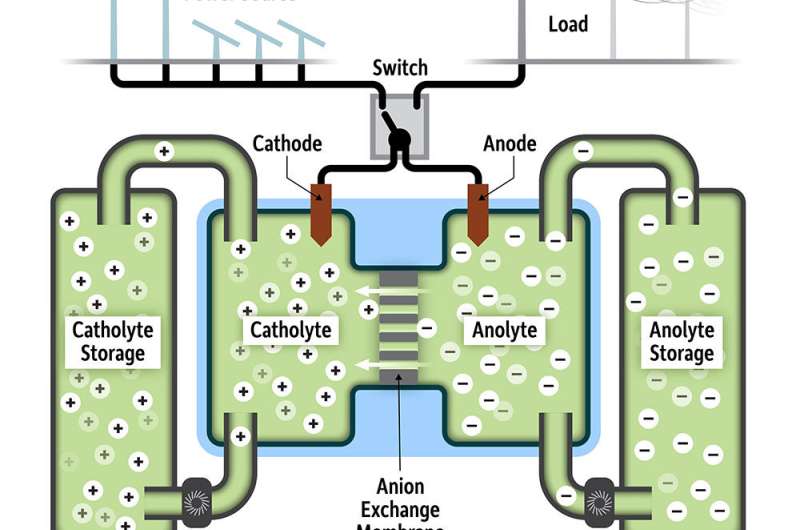
One possibility is to use any excess solar- and wind-based energy to charge solutions of chemicals that can subsequently be stored for use when sunshine and wind are scarce. During these down times, chemical solutions of opposite charge can be pumped across solid electrodes, thus creating an electron exchange that provides power to the electrical grid.
The key to this technology, called a redox flow battery, is finding chemicals that can not only "carry" sufficient charge, but also be stored without degrading for long periods, thereby maximizing power generation and minimizing the costs of replenishing the system.
Researchers at the University of Rochester and University at Buffalo believe they have found a promising compound that could transform the energy storage landscape.
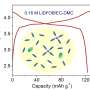
In a paper published in Chemical Science, an open access journal of the Royal Society of Chemistry, the researchers describe modifying a metal-oxide cluster, which has promising electroactive properties, so that it is nearly twice as effective as the unmodified cluster for electrochemical energy storage in a redox flow battery.

The research was led by the lab of Ellen Matson, PhD, University of Rochester assistant professor of chemistry. Matson's team partnered with Timothy Cook, PhD, assistant professor of chemistry in the UB College of Arts and Sciences, to develop and study the cluster.
"Energy storage applications with polyoxometalates are pretty rare in the literature. There are maybe one or two examples prior to ours, and they didn't really maximize the potential of these systems," says first author Lauren VanGelder, a third-year PhD student in Matson's lab and a UB graduate who received her BS in chemistry and biomedical sciences.
"This is really an untapped area of molecular development," Matson adds.
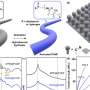
The cluster was first developed in the lab of German chemist Johann Spandl, and studied for its magnetic properties. Tests conducted by VanGelder showed that the compound could store charge in a redox flow battery, "but was not as stable as we had hoped."
However, by making what Matson describes as "a simple molecular modification"—replacing the compound's methanol-derived methoxide groups with ethanol-based ethoxide ligands—the team was able to expand the potential window during which the cluster was stable, doubling the amount of electrical energy that could be stored in the battery.

Cook's team—including fourth-year PhD candidate Anjula Kosswattaarachchi—contributed to the research by carrying out tests that enabled the scientists to determine how stable different cluster compounds were.
"We carried out a series of experiments to evaluate the electrochemical properties of the clusters," Cook says. "Specifically, we were interested in seeing if the clusters were stable over the course of minutes, hours, and days. We also constructed a prototype battery where we charged and discharged the clusters, keeping track of how many electrons we could transfer and seeing if all of the energy we stored could be recovered, as one would expect of a good battery.
"These experiments let us calculate the efficiency of the device in a very exact way, letting us compare one system to another. Because of these studies, we were able to make molecular changes to the cluster and then determine exactly what properties were effected."
Says Matson: "What's really cool about this work is the way we can generate the ethoxide and methoxide clusters by using methanol and ethanol. Both of these reagents are inexpensive, readily available and safe to use. The metal and oxygen atoms that compose the remainder of the cluster are earth-abundant elements. The straightforward, efficient synthesis of this system is a totally new direction in charge-carrier development that, we believe, will set a new standard in the field."
Matson and Cook's research groups have applied for a National Science Foundation grant as part of an ongoing collaboration to further refine the clusters for use in commercial redox flow batteries.
More information: L. E. VanGelder et al, Polyoxovanadate-alkoxide clusters as multi-electron charge carriers for symmetric non-aqueous redox flow batteries, Chemical Science (2018). DOI: 10.1039/C7SC05295B