Stopping fires before they start—how a salty solution is giving lithium metal batteries a safety check
Researchers have long considered lithium metal batteries to be the "holy grail" for energy storage. They have high energy density—how much energy a battery carries relative to its weight. This means they can be made smaller and lighter, while storing the same amount of energy as larger, heavier batteries made from other materials, or they can carry more energy in the same size battery.
Packing more energy into the same size battery means an electric vehicle using lithium metal batteries can drive farther on a single charge. In fact, batteries with a lithium metal anode have the potential to more than double the energy density of current electric vehicle batteries. But, among other performance improvements, they must first be made safer to use.
A Pacific Northwest National Laboratory research team has addressed safety as well as performance challenges posed by lithium metal batteries through the development of a new electrolyte. The electrolyte in a battery is the chemical solution that allows the electrical flow between the anode and cathode. The new electrolyte is described in the article "High-Efficiency Lithium Metal Batteries with Fire-Retardant Electrolytes," published in Joule.
Finding the "solution" to prevent fires
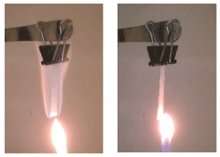
The main safety challenge with lithium metal batteries involves spikes or strands, called dendrites, of lithium that grow on the battery's anode. Dendrites can drain the battery's power, short its internal circuits, and impact the battery recharging capabilities. In some cases, dendrites have spontaneously combusted and caught fire.
To lessen or eliminate these safety and performance challenges, the team replaced components in an electrolyte containing a localized highly concentrated salt (lithium bis(fluorosulfonyl)imide) with a flame-retardant inert—or chemically inactive—material, triethyl phosphate/bis(2,2,2-trifluoroethyl) ether.
The combined solution forms highly-concentrated salt clusters that coat the anode with a layer of lithium deposits, eliminating dendrite formation and extinguishing the safety concerns.
A salty, yet steady, performance
The coating does not hurt the performance of the lithium metal anode, which has high efficiency (99.2 percent).
"The safe and stable high performance of this battery shows that we are one step closer to using lithium metal batteries in practical applications for electric vehicles," said Ji-Guang (Jason) Zhang, a battery expert and Laboratory Fellow at PNNL. "These findings may also help the development of similar, less expensive electrolytes to improve the performance and safety of other battery types."
More information: Shuru Chen et al. High-Efficiency Lithium Metal Batteries with Fire-Retardant Electrolytes, Joule (2018). DOI: 10.1016/j.joule.2018.05.002
Journal information: Joule
Provided by Environmental Molecular Sciences Laboratory