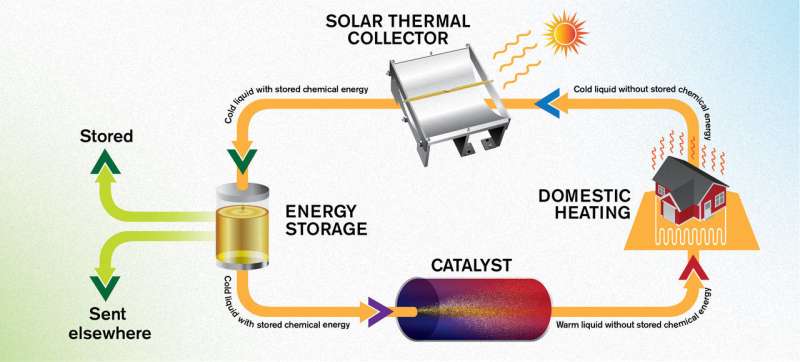
The energy system MOST works in a circular manner. First, the liquid captures energy from sunlight, in a solar thermal collector on the roof of a building. Then it is stored at room temperature, leading to minimal energy losses. When the energy is needed, it can be drawn through the catalyst so that the liquid heats up. It is envisioned that this warmth can then be utilised in, for example, domestic heating systems, after which the liquid can be sent back up to the roof to collect more energy -- all completely free of emissions, and without damaging the molecules. Credit: Yen Strandqvist/Chalmers University of Technology
A research group from Chalmers University of Technology, Sweden, has made great strides toward the development of a specially designed molecule which can store solar energy for later use. These advances have been presented in four scientific articles, the most recent appearing in Energy & Environmental Science.
Around a year ago, the research team presented a molecule that was capable of storing solar energy. The molecule, made from carbon, hydrogen and nitrogen, has the unique property that when it is hit by sunlight, it is transformed into an energy-rich isomer—a molecule consisting of the same atoms, but bound together in a different way.
This isomer can then be stored for use when that energy is later needed—for example, at night or in winter. It is in a liquid form and is adapted for use in a solar energy system, which the researchers call the Molecular Solar Thermal Energy Storage (MOST) system. In the last year, the researchers have made great advances in the development of MOST.
The research group developed a catalyst for controlling the release of the stored energy. The catalyst acts as a filter, through which the liquid flows, creating a reaction that warms the liquid by 63 degrees Celsius. If the liquid has a temperature of 20 degrees C when pumped through the filter, it comes out the other side at 83 degrees C. At the same time, it returns the molecule to its original form, so that it can be then reused in the warming system.
"The energy in this isomer can now be stored for up to 18 years. And when we extract the energy and use it, we get a warmth increase greater than we dared hope for," says the leader of the research team, Kasper Moth-Poulsen, professor at the Department of Chemistry and Chemical Engineering.
Professor Kasper Moth-Poulsen at the solar thermal collector, situated on the roof of the MC2 building at Chalmers University of Technology. Credit: Johan Bodell/Chalmers University of Technology
During the same period, the researchers also learned to improve the design of the molecule to increase its storage abilities so that the isomer can store energy for up to 18 years. This was a crucial improvement, as the focus of the project is primarily chemical energy storage. Furthermore, the system was previously reliant on the use of the flammable chemical toluene. But now, the researchers have found a way to remove the potentially dangerous toluene and instead use just the energy-storing molecule.
These advances mean that the MOST system now works in a circular manner. First, the liquid captures energy from sunlight, in a solar thermal collector on the roof of a building. Then it is stored at room temperature, leading to minimal energy loss. When the energy is needed, it can be drawn through the catalyst so that the liquid heats up. It is envisioned that this warmth can then be utilised in domestic heating systems, after which the liquid can be sent back up to the roof to collect more energy—all completely free of emissions, and without damaging the molecule.
"We have made many crucial advances recently, and today, we have an emissions-free energy system that works all year around," says Kasper Moth-Poulsen.
Professor Kasper Moth-Poulsen holding a tube containing the catalyst, in front of the ultra-high vacuum setup that was used to measure the heat release gradient in the molecular solar thermal energy storage system. Credit: Johan Bodell/Chalmers University of Technology
The solar thermal collector is a concave reflector with a pipe in the centre. It tracks the sun's path across the sky and works in the same way as a satellite dish, focusing the sun's rays at the point where the liquid leads through the pipe. It is even possible to add on an additional pipe with normal water to combine the system with conventional water heating.
The next steps for the researchers are to combine everything into a coherent system. "There is a lot left to do. We have just got the system to work. Now we need to ensure everything is optimally designed," says Kasper Moth-Poulsen. The group is satisfied with the storage capabilities, but more energy could be extracted, Kasper believes. He hopes that the research group will shortly achieve a temperature increase of at least 110 degrees Celsius and thinks the technology could be in commercial use within 10 years.
More information: Zhihang Wang et al, Macroscopic heat release in a molecular solar thermal energy storage system, Energy & Environmental Science (2018). DOI: 10.1039/C8EE01011K
Ambra Dreos et al. Liquid Norbornadiene Photoswitches for Solar Energy Storage, Advanced Energy Materials (2018). DOI: 10.1002/aenm.201703401
Martyn Jevric et al. Norbornadiene-Based Photoswitches with Exceptional Combination of Solar Spectrum Match and Long-Term Energy Storage, Chemistry - A European Journal (2018). DOI: 10.1002/chem.201802932
Mads Mansø et al. Molecular solar thermal energy storage in photoswitch oligomers increases energy densities and storage times, Nature Communications (2018). DOI: 10.1038/s41467-018-04230-8
Journal information: Energy & Environmental Science , Advanced Energy Materials , Chemistry – A European Journal , Nature Communications
Provided by Chalmers University of Technology