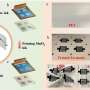
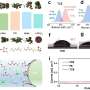
Demonstration of a renewable energy storage system. Photos of the researchers' demonstration of a renewable energy storage system based on a DZMB pack (in the middle of the photo) integrated with solar photovoltaic (PV) modules and a wind-driven generator through a controller. The controller was also connected to a light-emitting diode (LED) panel, serving as the electrical load. Credit: Zhong et al.
The global demand for rechargeable batteries has grown exponentially over the past decade or so, as they are needed to power the increasing numbers of portable electronic devices such as smart phones, laptops, tablets, smart watches and fitness trackers. To work most efficiently, rechargeable batteries should have a high energy density, yet they should also be safe, stable and environmentally friendly.
While lithium-ion batteries (LIBs) are now some of the most widespread rechargeable energy storage systems, they contain organic electrolytes that are highly volatile, which significantly reduces their safety. In recent years, researchers have thus been trying to identify new battery compositions that do not contain flammable and unstable electrolytes.
Among the most promising alternatives to LIBs are batteries based on non-flammable and low-cost water-based electrolytes, such as lead-acid and zinc-manganese batteries. These batteries have numerous advantages, including greater safety and low production costs. So far, however, their performance, working voltage and rechargeability have been somewhat limited compared to those of lithium-based solutions.
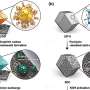
Researchers at the Key Laboratory of Advanced Ceramics and Machining Technology, the Tianjin Key Laboratory of Composite and Functional Materials and Tianjin University in China have recently introduced a new design strategy that could enhance the performance of zinc-manganese dioxide (Zn–MnO2) batteries. The approach they developed, presented in a paper published in Nature Energy, entails decoupling electrolytes inside the battery to enable an optimal redox chemistry in both Zn and MnO2 electrodes.
"Our paper occurred unintentionally when we assembled an alkaline Zn–MnO2 battery with freshly electrodeposited MnO2, which has some residual H2SO4 (from the electrodeposition bath) on the MnO2 surface," Prof. Cheng Zhong, one of the researchers who carried out the study, told TechXplore. "The assembled battery exhibited extra higher discharge voltage than conventional Zn–MnO2 batteries, which encouraged us to strip things down to basics, laying the foundations for our study."
Prof. Zhong and his colleagues found that their strategy for decoupling electrolytes led to better performing Zn-MnO2 batteries with an open-circuit voltage of 2.83 V. This is a highly promising result, considering that more conventional Zn-MnO2 batteries typically have a voltage of 1.5V.
The capacity of the battery fabricated using their electrolyte-decoupling strategy, dubbed DZBM, faded by just 2% after it was continuously used and recharged for 200 hours. In addition, the battery retained 100% of its capacity at a variety of discharge current densities. Remarkably, the researchers demonstrated that batteries created using their method can also be integrated with wind and photovoltaic hybrid power systems, which further increases their sustainability.
"The electrolyte-decoupling strategy aims at simultaneously enabling the optimal redox chemistry of both the Zn and MnO2 electrodes," Prof. Zhong explained. The working conditions of the MnO2 cathode and Zn anode were decoupled to enable both acidic MnO2 and alkaline Zn redox reactions in a single cell. The resulting DZMB battery has a much higher working voltage and prolonged cycling life than traditional alkaline Zn-MnO2 batteries."
In the future, the new design strategy introduced by Prof. Zhong and his colleagues could be used to produce new Zn-MnO2 batteries that are low-cost and safe, but that also have exceptionally high open-circuit voltages and a prolonged cycling life. Notably, the same strategy could also be used to enhance the performance of other zinc-based aqueous batteries, including those with Zn-Cu and Zn-Ag compositions.
"Since the cost and performance of state-of-art ion-selective membranes are still unsatisfactory, our future researches will focus on the studies of decoupling designs without using the membranes," Prof. Zhong said.
More information: Cheng Zhong et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries, Nature Energy (2020). DOI: 10.1038/s41560-020-0584-y
Journal information: Nature Energy
© 2020 Science X Network