Building a circular chemical economy

Carbon dioxide is essential to plant and animal life, but in excess it negatively impacts the environment by absorbing and radiating heat in the atmosphere, contributing to global warming.
But what if we could recycle carbon dioxide by converting it into useful fuels and chemicals? The University of Pittsburgh's James McKone is tackling this idea and was selected as a Beckman Young Investigator (BYI) by the Arnold & Mabel Beckman Foundation for this work.
"Over the last several decades, the cost of renewable electricity has dramatically decreased to the point where building a new solar or wind farm is, in many cases, more economical than continuing to run a coal-fired power plant," said McKone, assistant professor of chemical engineering at Pitt's Swanson School of Engineering.
"This is incredibly exciting because it means we can start to imagine what it would look like to power our whole society with carbon-free resources," he said.
Consider chemical manufacturing—the industry that produces most of the stuff that we use every day. The dangerous by-products and waste created by this industry adds to the massive global pollution problem—from the atmosphere to the depths of the ocean, and from backyards to beaches. According to McKone, simply improving renewable electricity is not enough to mitigate our climate impact if we do not also rethink the way we make things like plastic, steel, and textiles.
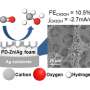
He received funding from the BYI program to develop new catalysts and chemical reactors that can recycle carbon dioxide and other chemical wastes back into useful fuels and raw materials.
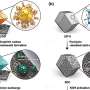
"We ultimately want to build a circular chemical economy—a sustainable approach to chemical manufacturing where every molecule that comes out of a smokestack or a tailpipe is captured and reused hundreds or thousands of times instead of being discarded as waste," said McKone.
His team will make two major adaptations to current industrial catalysts. Rather than heat, they will use electricity to drive chemical reactions so that they can use renewable resources as the main energy input. They will also mimic the behavior of biological enzymes to improve the efficiency of chemical reactions by designing specific catalytic units, called active sites, to perform each individual step of the complex chemical reactions.
"Getting these catalysts to work is an incredible challenge," said McKone. "To meet that challenge, we are developing new experimental capabilities that will allow us to measure and manipulate catalyst materials with atomic-scale precision."
The BYI program provides research support to the most promising young faculty members in the early stages of their academic careers in the chemical and life sciences. It challenges researchers to pursue innovative and high-risk projects that seek to make significant scientific advancements and open up new avenues of research in science.
McKone is only the second Pitt professor selected for this award in the history of the BYI program. The first was Steven Little, William Kepler Whiteford Endowed Professor and Chair of Chemical and Petroleum Engineering. Alex Deiters, professor of chemistry at Pitt, is a third BYI who received the award during his tenure at North Carolina State University.