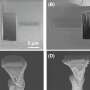
In a measuring cell developed in-house, the Oldenburg team examined lithium electrodes using electrochemical scanning microscopy. Credit: Bastian Krueger
What happens inside a battery at the microscopic level during charging and discharging processes? A team of scientists led by Prof. Dr. Gunther Wittstock of the University of Oldenburg's Chemistry Department recently presented a new technique for live observation of processes that until now have been largely unobservable in the scientific journal ChemElectroChem.
According to the researchers, this new technique could accelerate the search for suitable materials for innovative batteries, with the ultimate objective of developing eco-friendlier energy storage devices that are more durable and have a higher power density. Scientists from the battery research center MEET (Münster Electrochemical Energy Technology) at the University of Munster are also on the team.
Batteries convert chemical energy into electrical energy. During this process, charged particles cross from a positively charged electrode, the cathode, to the negative anode. In many modern batteries and rechargeable batteries, the reactive metal lithium is an important component of the anode. During operation, ultra-thin layers form on the surface which protect both the electrode and the battery fluid from decomposition. Until now, however, it has been almost impossible to directly observe the changes that take place in these complex layers—just a few millionths of a meter (micrometers) thick—during charging and discharging cycles.
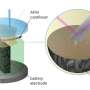
The team has developed a new measuring principle to obtain local, high-resolution information about the surface of metallic lithium electrodes during battery operation. "Over time, chemical processes on the electrode's surface can have a major impact on the durability and performance of a battery," said Wittstock.
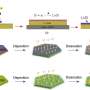
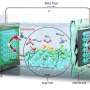
The researchers used scanning electrochemical microscopy (SECM) for their analysis. This procedure involves scanning a measuring probe across the surface of a sample to collect chemical information at intervals of a just a few micrometers. Special software then translates the measured data into a colored image. "By repeating this process several times we can track changes on the sample's surface like in a flipbook," Wittstock explained.
Bastian Krueger, a Ph.D. student of Wittstock's Physical Chemistry research group, developed a special measuring cell in which experimental conditions—such as current intensity—essentially corresponded to those in a real battery. The chemist tested various cell assemblies which he produced using 3-D printers and CNC micro-milling machines. Luis Balboa, another Ph.D. student of the same group, carried out computer simulations to optimize the cell geometry that recreate realistic experimental conditions. The team from Munster contributed reference samples.
With this setup, the scientists were able to observe the processes on the lithium anode with an unprecedented degree of accuracy. They observed how, at high charging speeds, lithium from the battery fluid was deposited on the anode. These locally reinforced deposits can develop into so-called dendrites—branching extensions of lithium on the electrode. Such formations limit the durability of batteries and in extreme cases can even cause their destruction.
"The breakthrough in our study consists in the fact that for the first time ever we were able to carry out such processes at realistic current densities directly within the measuring apparatus and visually monitor their effects," Wittstock stressed. The technique could also be used on other types of electrodes, he added, explaining that the long-term objective was to study how different pre-treatment steps influence the formation of a protective boundary layer on electrodes.
More information: Bastian Krueger et al, Solid Electrolyte Interphase Evolution on Lithium Metal Electrodes Followed by Scanning Electrochemical Microscopy Under Realistic Battery Cycling Current Densities, ChemElectroChem (2020). DOI: 10.1002/celc.202000441
Provided by University of Oldenburg