When it comes to battery dendrite formation, a negative number yields positive results

Consumer demand has driven engineers to design batteries that are more compact while at the same time maintaining, or even improving, their capacity.
On paper, it seems there is plenty of room to increase battery capacity without increasing the charging current density to the critical point—called the limiting current density—beyond which a battery can short circuit.
Yet batteries working at current densities—the amount of charge that passes through a particular area in a defined amount of time—well below their established thresholds have been known to fail.
Now research from the lab of Peng Bai, assistant professor of energy, environmental and chemical engineering at the McKelvey School of Engineering, has resulted in the ability to not just approximate the threshold, but to accurately predict the short circuit time for any particular current density.
The findings were published online in August in the journal Energy & Environmental Science.
Not only have the researchers described this relationship, but they have been able to express it in terms of a single number. "This one number defines the relationship between the most important physical quantities, current density and short-circuiting time," said first author Youngju Lee, a doctoral student in Bai's lab. Another doctoral student, Bingyuan Ma, was also on the research team.
There are multiple ways a lithium battery can fail, but a longstanding problem associated with high currents is dendrite penetration. Dendrites are tree-like structures that can form on the lithium plating in a battery. They can quickly penetrate a battery's ceramic separator, a porous plastic film between the anode and cathode of the battery. Once a dendrite makes its way across the separator, the battery shorts out.
The growth of dendrites depends on the current density, but dendrites spring up in batteries at current densities three orders of magnitude higher than expected or designed.
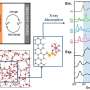
In previous studies of battery failures, researchers would open a battery and see black spots on the separator, indicating the locations where dendrites had penetrated the separator.
When Lee did the same, and analyzed the area covered by spots, they turned out to be 0.1 percent of the total area of the separator, which meant the current responsible must have been 1,000 times higher than expected.
Already, there was the puzzle as to how batteries were shorting out despite having not reached that critical current density—they had, locally.
"When designing batteries, we use the entire area of the electrode or separator to calculate the current density," Bai said. But whatever is causing the high current density is happening locally.
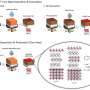
Using a unique, transparent capillary cell developed in Bai's lab, they were able to watch the dynamics of lithium metal growth , reconstructing the actual metal penetration dynamics through separator pores. "We found it was more complicated than we thought," Bai said. "It's very sensitive to the geometry of the channel."
Essentially, they found that single electrolyte channels with converging cross-sectional areas, and effectively practical separators that may induce fewer and fewer penetrated pores in the metal growth direction will accelerate and exacerbate the dynamics and lead to faster short circuiting.
"But if you have an expanding channel, you can delay and even avoid the formation of dendrites," Lee said. "That's what we discovered. And how do we describe these channels?"
With a single number.
If that number (an exponent in Sand's formula) is less than -2 then you've got a safer lithium ion battery. But if it's higher than -2, for example -1.5, that's no good, Bai said. "Then your battery will short circuit sooner than is predicted by the standard model widely used in battery design."
"It's always been easy to blame dendrite penetration for battery failures," Bai said. "But what is more important is to thoroughly understand the dynamics in practical conditions. Yet what has never been done is being able to consistently predict all short circuit times for all current densities.
"We found a way—not just to do it 'more accurately,' but now we can make predictions because we know the true physics of the system. And because of that, we can rely on this single number, the Sand's time exponent, to assess the safety level of separators and guide the optimization to achieve a number less than -2, such that we can avoid dendrite penetration all together."
More information: Youngju Lee et al. Concentration polarization and metal dendrite initiation in isolated electrolyte microchannels, Energy & Environmental Science (2020). DOI: 10.1039/D0EE01874K