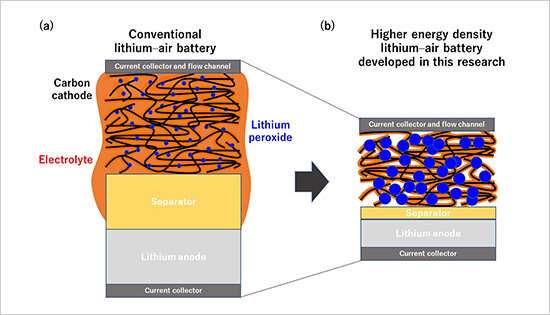
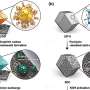
Figure 1. Schematic diagrams of an existing Li-O2 battery and the higher energy density Li-O2 battery developed in this research. Credit: National Institute for Materials Science
NIMS and SoftBank discovered that the cycle life of high energy density lithium-air batteries is predominantly influenced by the ratio of electrolyte volume to electrode areal capacity. The team developed a technique to quantify side reaction products formed and oxygen used in battery electrochemical reactions. Using this technique, the team was able to precisely assess the overall balance between reactants and products, leading to this discovery. These results may facilitate R&D concerning practical lithium-air batteries.
Lithium-air batteries have potential to develop into the ultimate rechargeable batteries: they are lightweight and have high energy capacity and their theoretical energy density is several times the energy density of current lithium-ion batteries. Because of these potential advantages, they are anticipated to be applicable to a wide range of technologies, such as drones, electric vehicles and household electricity storage systems. The NIMS-SoftBank Advanced Technologies Development Center, established in 2018, has been carrying out lithium-air battery research to achieve the practical use of this battery in various technologies, including mobile phone base stations, the internet of things and HAPS (high altitude platform stations). Extending battery cycle life is particularly important for these purposes. Most evaluations of lithium-air battery cycle life had focused on assessment of individual battery components, such as electrode materials, and only a few studies have taken an approach of actually fabricating functional high energy density lithium-air batteries and evaluating their cycle lives. In addition, only a few methods for quantitively measuring substances involved in battery electrochemical reactions (e.g., oxygen used as the cathode active material and gaseous by-products) were available. Because of these limitations, overall balance between reactants and products was impossible to measure, making it difficult to identify primary factors influencing battery cycle life and slowing research and development of practical lithium-air batteries.
This research team recently developed a technique capable of quantifying oxygen used in electrochemical reactions and gases and volatile substances produced during charge and discharge cycles. The team then used this technique to precisely analyze complex electrochemical reactions occurring in high energy density lithium-air batteries developed by the NIMS-SoftBank Advanced Technologies Development Center. As a result, the team found for the first time that the cycle lives of lithium-air batteries are predominantly influenced by the ratio of electrolyte volume to electrode areal capacity. This means that battery cycle life can be extended by decreasing the areal capacity of the electrode while maintaining the volume of the electrolyte constant. However, decreasing electrode areal capacity will also cause the energy density of the battery to decrease. The development of practical lithium-air batteries will therefore require optimization of the ratio parameter during the process of designing batteries and evaluating battery materials.
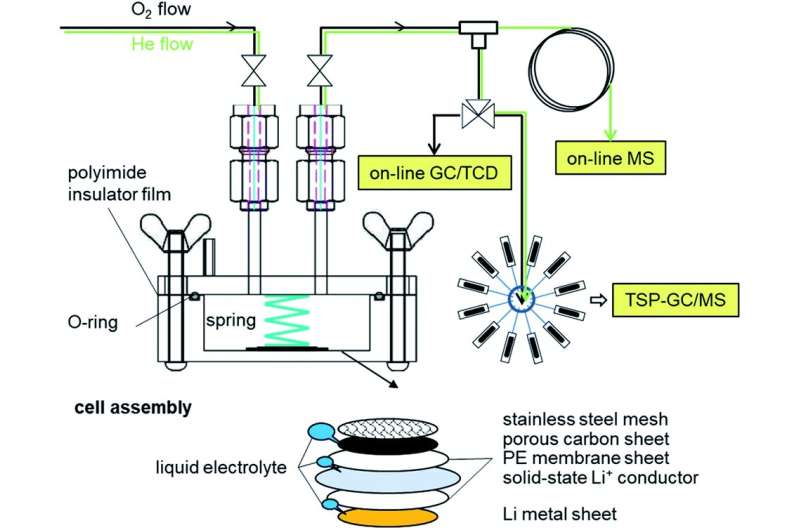
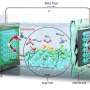
A Li–O2 two-compartment test cell and characterization tools for evolved gases. DOI : 10.1039/d0ra07924c
In future research, we intend to develop methods for reducing the amounts of by-products produced by electrochemical reactions in batteries, thereby accelerating the efforts of the NIMS-SoftBank Advanced Technologies Development Center to put lithium-air batteries into practical use.
This research was published in RSC Advances, an online journal, on December 2, 2020.
More information: Makoto Ue et al. Material balance in the O2 electrode of Li–O2 cells with a porous carbon electrode and TEGDME-based electrolytes, RSC Advances (2020). DOI: 10.1039/d0ra07924c
Provided by National Institute for Materials Science