Jeroen van Oijen. Credit: Bart van Overbeeke
The combustion engine—a thing of the past? Think again! Researchers at Eindhoven University of Technology believe they have found a way to make this unsustainable power source ready for the future. The secret: the noble gas argon. Their main challenge now is to find a way to ignite the gas mixture at just the right moment. Or as Jeroen van Oijen, researcher at the department of Mechanical Engineering of TU/e, puts it: "We want to know when to push the swing."
Combustion engines, both internal (diesel and gasoline) or external (steam), have long been the drivers of the modern world as we know it. However, environmental concerns about harmful CO2 and nitrogen oxide emissions have transformed this fossil fuel-driven engine into a suspect legacy, often mentioned in the same breath as coal-fired power stations. It explains why many researchers working on sustainable power generation want to look beyond combustion as a means of producing energy.
Jeroen, why do you believe it's too early to write off the combustion engine?
We at TU/e strongly believe that we can revolutionize the combustion engine for the 21st century. To achieve this, we are exploring a number of clean revisions for the combustion engine. One promising avenue is the argon power cycle (APC). APC is a groundbreaking technology, which uses argon instead of air as the working fluid. Combine that with hydrogen as your fuel, and you have the potential for a highly efficient machine, that is not only emission-free, but can also help store green energy from solar and wind.
Why argon?
Argon is a noble gas, which means that it doesn't react with other gases. But, more importantly, it is mono-atomic: it consists of only one atom. This is a key advantage over air, which consist mainly of diatomic molecules like nitrogen and oxygen. If you compress air, as you do in a normal combustion engine, the molecules in the air start to vibrate and rotate. This leads to the loss of some of the input energy, which is stored in the molecules as internal energy, instead of all the energy being used to maximize the kinetic energy needed to push the piston. By using argon all input energy is converted into internal pressure in the piston cylinder. This means the efficiency can be increased by about 25 percent, reaching values above 80 percent!
You may wonder why we are still using air in the engines of our motor vehicles if argon is such a magical gas. Well, air is abundant. It's all around us. But, more importantly, it already includes the oxygen you need to burn the fuel and convert the chemical energy into heat. If you switch to argon, you have to inject the oxygen separately.
Air consists to 1 percent of argon. It is therefore the cheapest and most widely used noble gas. In this photo we see a vial of glowing ultrapure argon. Credit: Eindhoven University of Technology
And where would you get the argon?
Argon is widely available in air and can be cheaply extracted from air as a side product of cryogenic air separation. What's more: you only need to remove argon from air once. Since argon is a noble gas, it goes through the combustion process without reacting with other gases, so at the end you have argon once again. The only thing you need to do is cool it down to remove the water that results from the combustion process. Once you've introduced it into a closed system, it can be recycled again and again, in an endless closed loop.
What else do you need besides argon?
As I said, we want to use hydrogen as a fuel, instead of fossil fuels like gasoline or diesel. Hydrogen has two main advantages: when it reacts with oxygen, you get plain old water as an end product, instead of harmful CO2 and NO. Secondly, and this is really key: hydrogen is a very promising storage material for green energy. This means you have sustainable energy at your disposal whenever you need it, and not just when there's sun and wind!
I understand there are still quite some practical hurdles to overcome before we have a fully working and efficient APC engine. What do you see as the main challenges for this research project?
As I said, to get the combustion started you need to introduce fuel and oxygen into the combustion system. This is the kick to get everything going. Ideally, you want to inject the fuel and oxygen when the argon is fully compressed, as this gives you the best efficiency. But practically, it is easier to inject the reactants before compression. However, because argon tends to heat up very rapidly when it's compressed, such a mixture ignites before you've reached the optimal pressure. Obviously, this affects the efficiency of the system. To understand the problem, imagine a swing on a playground: you want to push precisely at the moment that your efforts create the most momentum, and not one second earlier or later. The same is true for the piston in the combustion engine.
So you need new techniques to control the combustion process in an argon engine?
Exactly. In the research project we want to explore three possible solutions. The first one appears to be the most promising, as it resembles the method used in a diesel engine. It involves introducing the hydrogen only after the argon and the oxygen have been fully compressed. This avoids premature ignition, but there are still some problems to solve. It turns out that it's quite hard to inject hydrogen into a compressed gas, because it is extremely light.
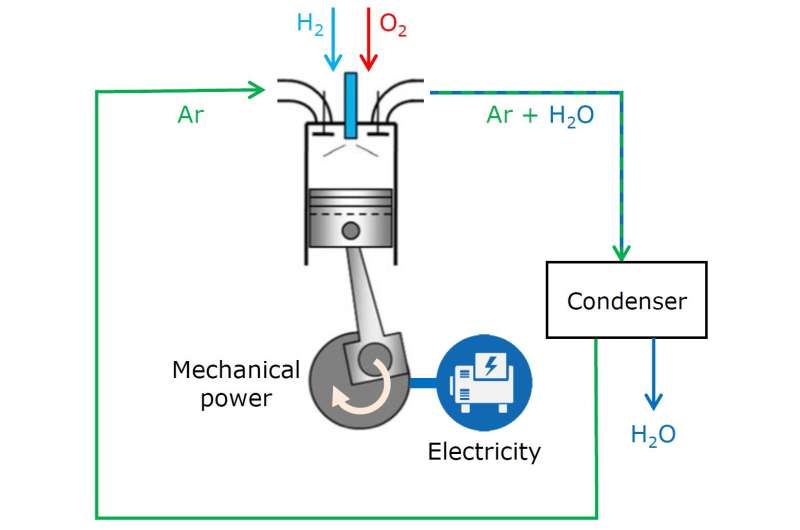
Schematic overview of the Argon Power Cycle with hydrogen as fuel. Credit: Eindhoven University of Technology
A second alternative we want to explore, solves this problem by injecting hydrogen and argon when the pressure is still low, and bringing in oxygen, a heavier gas than hydrogen, at a later stage when the pressure is high. The problem here is that this has never been tried before, and that oxygen at high pressure tends to react with the metal of the injector, leading to corrosion.
Finally, we want to look into the option of injecting both hydrogen and oxygen at a later stage in the cycle. Here the challenge is to align the injection of the two gases to ensure that both gases can find one another and react at the right time.
Suppose you are able to solve these challenges, and in five to 10 years' time, we'll have a combustion engine that is both highly efficient and free of CO2 emissions. What do you see as the main application of this engine?
First and foremost, we think that the APC engine will be used for the generation of electricity, using the power that is generated from wind and solar and stored in hydrogen. But our APC engine can also be used with natural gas or biofuels. Of course, it will then no longer be carbon-free, but the great thing about our set-up is that it is closed. This makes it much easier and cheaper to capture the CO2 emissions. These can then be used as an ingredient for the chemical industry. To filter out CO2 we use a sophisticated membrane that only has a limited effect on the overall efficiency of the system.
And cars and lorries?
I'm afraid our system will probably be less suited for road vehicles because of the relatively high heat loss in smaller engines. But we do see a future for it in settings where you need very large engines, like in ships.
Finally, does TU/e have any plans to bring the APC engine to the market?
As a matter of fact, I'm working together with a researcher in Berkeley, who has already begun a start-up to commercialize this product. His name is Miguel Sierra Aznar, and he used to be a MSc student of mine. His company is called Noble Thermodynamics. We are also working together with Winterthur Gas & Diesel to explore future market opportunities. But, of course, this is some way off for going to market. First, we have to get the science right, and make sure our APC engine is as clean and as efficient as possible!
Provided by Eindhoven University of Technology