March 24, 2021 feature
Investigating the role of cobalt in rechargeable batteries to develop more effective cobalt-free cathodes
Many automotive companies worldwide are currently investing in the development of electric vehicles, which could help to reduce greenhouse gas emissions. Electric vehicles require large rechargeable batteries that exhibit a high performance, durability and energy-efficiency.
In recent years, the development of electric vehicles was hindered and slowed down by the rising cost of rechargeable batteries. The main reason for this high cost is that cobalt (Co), which is the primary component of most cathodes in rechargeable batteries, has become harder to attain and is increasingly in demand.
To circumvent this problem and ensure that there are enough rechargeable batteries to power new electric vehicles and other technologies, engineers and chemists have been trying to identify alternative materials that could substitute Co in common cathodes for rechargeable batteries. In order to effectively substitute Co, these materials should achieve a similar performance and yet be more affordable or readily available.
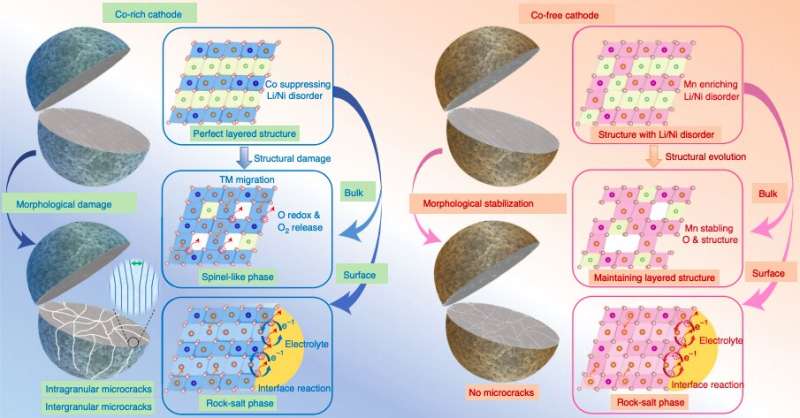
Researchers at Argonne National Laboratory and Peking University Shenzhen Graduate School in China have recently carried out a study aimed at better understanding the role that Co plays in rechargeable batteries, in order to inform the design of new Co-free cathodes. Their paper, published in Nature Energy, identifies some of the processes and characteristics that make cobalt advantageous for realizing highly performing rechargeable batteries.
"We investigate the roles of Co in purposely designed systems that include both Co-rich and Mn-substituted Co-free cathodes," the researchers wrote in their paper.
Many Co-free cathodes proposed so far are rich in Lithium (Li) or Magnesium (Mg). While some of these cathodes have been found to be fairly promising for replacing Co-based cathodes, their capacities and operational stability are often unsuitable for large-scale commercial use. As a result, most studies exploring Co-free alternatives focused on layered oxide cathodes, such as Nickel (Ni)-rich layered oxide cathodes.
Ni-rich layered oxide cathodes typically exhibit high capacities and energy densities. Nonetheless, some researchers found that directly replacing Co with Ni is unfeasible, as it can lead to significant reductions in a battery's performance and thermal stability.
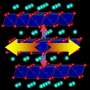
In their paper, Tongchao Liu and his colleagues at Argonne National Laboratory and Peking University tried to gain a better understanding of the reasons why Co plays such a vital role in cathodes for rechargeable batteries. Their hope was that this would help them to identify alternative Ni-rich compositions that achieve similar performances and design better Co-free cathodes for rechargeable batteries.
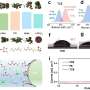
The researchers used state-of-the-art methods to identify the intrinsic properties of Co and compare its processes to those that take place in Ni-rich and Co-free cathodes. Past studies suggested that a high amount of Ni in a cathode may cause a battery's capacity to decay more rapidly. The findings gathered by Liu and his colleagues, however, show that Co is actually more destructive that Ni at high potentials.
"Our results affirmed that Co plays an undeniable role in fast capacity and/or structural degradation and found that Co is more destructive than Ni at high potentials, which offers unexpected but encouraging perspectives for Co reduction," the researchers explained in their paper. "Moreover, Mn substitution effectively alleviates the destructive effects of Co and enables a high potential functionality."
The results gathered by this team of researchers offer some valuable insight that could inform the design and realization of better performing, more stable and cost-effective cathodes for rechargeable batteries. Overall, the team identified a series of morphological and structural degradation mechanisms that could explain why the capacity of Co-rich cathodes decays quickly over time.
Building on these findings, Liu and his colleagues already started realizing a series of Co-free cathodes that achieved promising performances. In the future, these cathodes could be integrated in rechargeable batteries, which may then be commercialized and become widely available.
More information: Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nature Energy(2021). DOI: 10.1038/s41560-021-00776-y.
© 2021 Science X Network