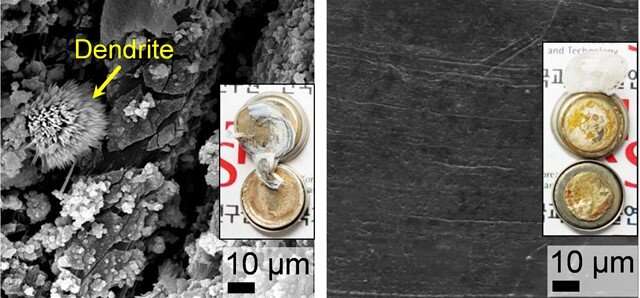
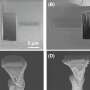
Top-view SEM images and photographs (inset) of plain-Li, and Li@p-PCL electrodes after cycling tests with Li|Li symmetrical cells at 1.0 mA cm-2 and 1.0 mAh cm-2. Credit: Korea Institute of Science and Technology (KIST)
Despite rapid development of electric vehicles (EVs), lithium-ion (Li-ion) batteries are a fire and explosion risk. Among the approaches to tackle this issue, Korean researchers have used semiconductor technology to improve their safety. A research team from the Korea Institute of Science and Technology (KIST) led by Dr. Joong Kee Lee of the Center for Energy Storage Research has succeeded in inhibiting the growth of dendrites, crystals with multiple branches that cause EV battery fires by forming protective semiconducting passivation layers on the surface of Li electrodes.
When Li-ion batteries are charged, Li ions are transported to the anode (the negative electrode) and are deposited on the surface as Li metal; at this point, tree-like dendrites form. These Li dendrites are responsible for the uncontrollable volumetric fluctuations and leads to reactions between the solid electrode and the liquid electrolyte, which causes a fire. Unsurprisingly, this severely degrades battery performance.
To prevent dendrite formation, the research team exposed plasma to fullerene (C60), a highly electronic conductive semiconductor material, resulting in the formation of semiconducting passivation carbonaceous layers between the Li electrode and the electrolyte. The semiconducting passivation carbonaceous layers allow Li-ions to pass through while blocking electrons due to generation of Schottky barrier, and by preventing electrons and ions from interacting on the electrode surface and inside, they stop the formation of Li crystals and the consequent growth of dendrites.
The stability of the electrodes with the semiconducting passivation carbonaceous layers was tested using Li/Li symmetric cells in extreme electrochemical environments where typical Li electrodes remain stable for up to 20 charge/discharge cycles. The newly developed electrodes showed significantly enhanced stability, with Li dendrite growth suppressed for up to 1,200 cycles. Moreover, using a lithium cobalt oxide (LiCoO2) cathode in addition to the developed electrode, approximately 81% of the initial battery capacity was maintained after 500 cycles, representing an improvement of approximately 60% over conventional Li electrodes.
KIST researchers are looking at plasma polymerized carbon semiconductors for lithium electrodes. Credit: Korea Institute of Science and Technology (KIST)
Lead researcher Dr. Joong Kee Lee said, "The effective suppression of dendrite growth on Li electrodes is instrumental for improving battery safety. The technology for developing highly safe Li-metal electrodes proposed in this study provides a blueprint for the development of next-generation batteries that do not pose a fire risk." Dr. Lee says his team's next goal is improving the commercial viability of this technology: "We aim to make the fabrication of the semiconducting passivation carbonaceous layers more cost-effective by substituting fullerene with less expensive materials."
More information: Ryanda Enggar Anugrah Ardhi et al, Metal–Semiconductor Ohmic and Schottky Contact Interfaces for Stable Li-Metal Electrodes, ACS Energy Letters (2021). DOI: 10.1021/acsenergylett.1c00150
Provided by National Research Council of Science & Technology