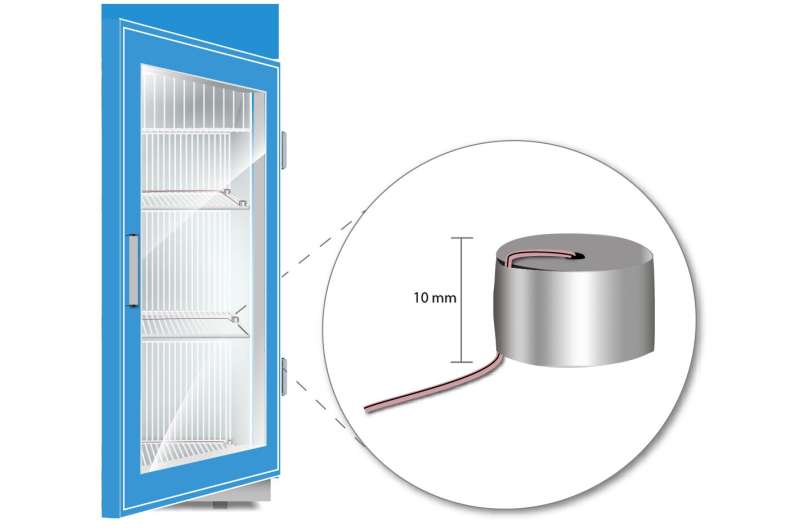
Vaccine fridges and freezers must now meet certain general criteria in order to be certified according to the new voluntary standard. Credit: Kristen Dill/NIST
You know that part of your fridge that always freezes your lettuce? Or the section in your freezer door that leaves your popsicles a little mushy?
Different parts of a food-grade commercial cooling unit might be colder or warmer, depending on how close they are to a wall, or whether the shelves are solid versus a more breathable wire mesh, for example.
Refrigerators used to store vaccines can have these problems too, but the cost of going outside the proper temperature range is more than a head of lettuce. One case study in Australia found that more than half of discarded vaccine doses were the result of mechanical failures in which components of the fridge malfunctioned. The design of the unit can sometimes result in these mechanical failures.
Some appliances are marketed specifically as "vaccine" or "medical" refrigerators. However, those terms are not regulated, and manufacturers can apply them to products with vastly different capabilities.
To get around this problem, vaccine providers implement their own sometimes complicated aftermarket engineering controls—for example, adding multiple thermometers so that temperature can be logged in different locations, or adding water bottles to improve temperature stability. But even minor mistakes can damage the stored vaccines.
"If we can improve our vaccine storage equipment, we can go a long way toward reducing the incidence of vaccine waste," said NIST researcher Michal Chojnacky.
To create a new set of voluntary standards that manufacturers can use to make vaccine storage equipment, NIST staff members have been serving on a committee led by the public health and safety organization NSF International (formerly the National Sanitation Foundation). (NSF International develops standards for the American National Standards Institute, ANSI, a private non-profit that oversees standards and conformity assessment activities in the United States.) This week, the committee published a baseline set of equipment performance requirements for the safe storage of vaccines.
The new standard applies to medical fridges and freezers with temperature ranges that could be used for most vaccines, including flu shots, almost all childhood vaccines, and even the COVID-19 vaccines, at least under some circumstances.
The committee also includes members from various stakeholders, including industry, government and public health groups.
"What we're trying to do is help people buy equipment that is fit to the purpose," Chojnacky said. There are already a lot of protocols and oversight in place, at least in the case of the federally funded Vaccines for Children program, she continued. But in many cases, it can be difficult for clinicians to find the right equipment.
"It's not their expertise to know exactly how refrigerators function," Chojnacky said. "So the purpose of the standard is to make lives easier for the people who are in charge of safely distributing vaccines."
To be clear, it is not mandatory for vaccine storage units to conform to the new standard. Manufacturers may voluntarily choose to comply with the requirements and submit their units for independent testing. Buyers who choose to purchase a unit certified to the NSF/ANSI standard will know that the equipment meets the requirements for safe vaccine storage without the need for aftermarket engineering controls.
It's "Standard' Time
The VSD (vaccine simulation device) illustrated here was designed by the standard committee as a stand-in for a real vaccine vial. Each device is small — only 10 mm tall — and made of aluminum. The devices will be used by laboratories to evaluate the performance of medical fridges and freezers being considered for NSF certification. Credit: Kristen Dill/NIST
In making the standard, the committee strove to ensure that certification requirements were feasible, not too expensive, and truly necessary, Chojnacky said.
For example, the range of acceptable temperatures for most vaccines is 2 to 8 degrees C (about 35 to 46 degrees F) for fridges and -50 to -15 degrees C (about -58 to 5 degrees F) for freezers. Built-in monitoring equipment might track temperatures in a single location within the unit, but that does not tell clinicians whether there are gradients or deviations from that temperature in other areas of the device.
Furthermore, if vaccines are pushed toward the back or sides of a unit, they might be cooler or warmer than acceptable—and the monitoring equipment wouldn't even tell anyone that there was a problem.
To avoid this issue, the new standard requires that units must make it physically impossible for vaccines to be put, even accidentally, into areas of the unit where the temperature cannot be kept within the acceptable range. The method of doing this is up to the manufacturer, but one way is to put mesh barriers around the inner sides of the fridge to block off areas that tend to get too hot or cold.
Other examples of certification requirements include self-closing doors and both visual and auditory warnings on the front of the unit if the temperature goes outside of the desired range.
Testing, Testing, 1-2
To give the committee real-world data to use as a basis for the standard, Chojnacky's team conducted several studies. (Another committee member, representing a manufacturer, also contributed data.) In one study, NIST researchers sent data logging instruments to vaccine clinics around the country to find out how many times a day vaccine fridge doors were opened, and for how long. They then used this data to come up with a test protocol that would simulate how the fridges are really used, which includes short door openings of eight seconds (likely for taking out doses to administer) and long door openings of three minutes (likely for stocking fridges and running inventory checks).
But one of the most important pieces of information that NIST and the other committee member contributed allowed the group to determine how the standard's certification testing process should work.
First, the committee needed to develop a "vaccine simulation device," or VSD—a stand-in for a vial of medication that would simulate what a real vaccine would experience, temperature-wise, in a fridge or freezer. Traditional vaccine stand-in objects are made of brass, but the committee found that tiny 10-millimeter-tall (a little smaller than half an inch) aluminum cylinders were more reliable for its needs.
"We needed the VSD to not only be representative of the vaccine temperature, but also easy to make without the need for any difficult-to-find, expensive material," Chojnacky said. A kind of thermometer called a thermocouple is embedded into each aluminum cylinder, which can then be placed in different areas around the fridge or freezer.
The testing protocol, which is also laid out in the new standard, will involve putting the VSDs into the manufacturer's unit to measure their temperatures under different conditions—for example, with frequent short door openings (a few seconds) or frequent long door openings (a few minutes). Some tests include filling the unit with empty boxes to obstruct air flow, to simulate a packed fridge.
"We needed to make sure our methodology could easily be replicated by an independent test lab," Chojnacky said. "NSF International has its own accredited lab doing the testing, but other labs can also get that accreditation and conduct their own tests for the certifications. We wanted to make sure the test is simple and repeatable, regardless of where it's performed."
Though the official standard is now published, it isn't yet clear how soon the certification testing process will be up and running. Committee members hope the tests will be available within the next several months.
Provided by National Institute of Standards and Technology