Chemists develop novel electrolyser for hydrogen production
In a recent Nature Communications paper, a group of researchers led by Dr. Ning Yan of the Van 't Hoff Institute for Molecular Sciences at the University of Amsterdam showcases a practical membrane-free approach to water electrolysis using earth-abundant catalysts. Their new electrolyser concept, developed together with researchers from Wuhan University and Wuhan University of Technology, offers significant advantages over electrolysers that are currently being developed for large-scale hydrogen production.
The transition to a hydrogen economy is a must for advancing sustainable energy practices as well as for tackling climate change. Hydrogen that is produced through water electrolysis using renewable electricity can be used both as a clean energy carrier and as a reagent for making bulk chemicals from CO2. Large-scale water electrolysis is an essential technology for realizing these goals. However, while electrolysers have been known for over 200 years, the technology is still facing major challenges. For instance, the conventional alkaline electrolysis is more suitable to operate at low current density and low pressure, while the emerging proton exchange membrane (PEM) electrolyser requires the use of scarce noble metal catalysts and extensive water purification.
Sandwich-like architecture
Now, a group of researchers led by Dr. Ning Yan from the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences present a new type of membrane-free electrolyser that can split water into hydrogen and oxygen at high current density using only earth-abundant catalysts. The work, performed together with researchers from Wuhan University and Wuhan University of Technology, has recently been presented in a paper in Nature Communications.
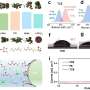
The new electrolyser comprises of two identical and separate compartments with a sandwich-like architecture. Through this sandwich flow two solutions: a hydrogen-rich catholyte and an oxygen-rich anolyte. During operation, the anolyte and catholyte cycle back and forth so that the roles of each compartment are continuously reversed. As a result, the novel electrolyser delivers hydrogen gas of over 99% purity.
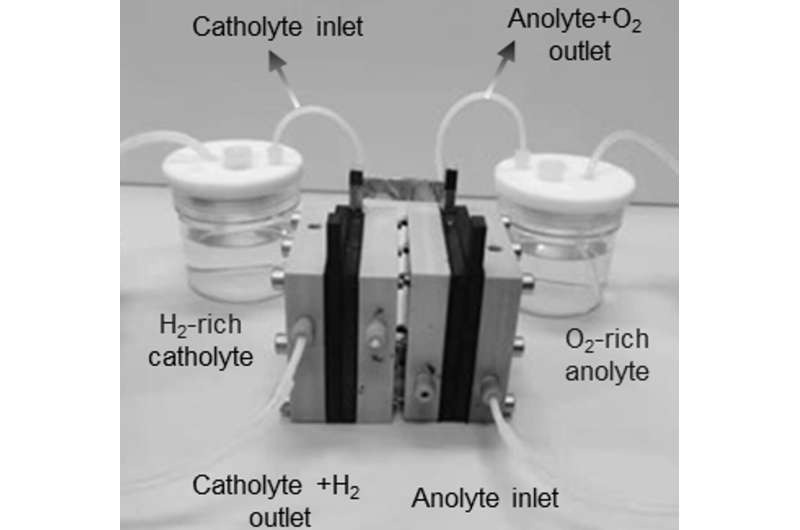
According to Dr. Yan, this new setup combines the best of both worlds: "The closely packed sandwich structure results in a short travel distance of ions, making the ohmic resistance of our membrane-free cell comparable with that of a PEM electrolyser. Together with the separation of the two reaction chambers, this opens opportunities for the cell to work at high current densities that are comparable with those of PEMs. Moreover, our electrolyser design is very robust, and works equally well both in deionized water and in regular tap water."
Cyclic operation
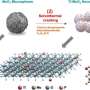
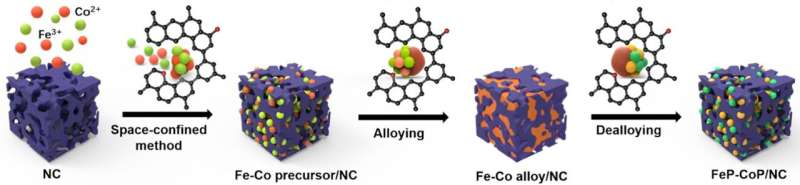
To enable continuous performance, the electrolyser is operated in a cyclic way where the electrode catalyst is bifunctionally active. Tests have revealed that it performs equally well in both the water reduction reaction and water oxidation reaction. An important advantage here is that no noble metals are needed. Instead, the cell uses a modified version of the nitrogen-doped catalysts that have been developed earlier by Yan and Prof. Gadi Rothenberg for fuel cell and supercapacitor applications. These highly porous and structured materials have now been used by Ph.D. student Jasper Biemolt as supports for iron-cobalt alloys and their phosphide derivatives (see first image).
Rothenberg explains that using earth-abundant materials holds the key to real-life applications: "To compete effectively in the market, the cost of green hydrogen should be below 2 euro per kilogram. This means that commercial large-scale production of hydrogen needs to find alternative solutions. By designing electrolysers with new configurations and using catalysts based on abundant elements, we create the possibility for real-life implementation."
Yan and Rothenberg are aware that scaling up this cell technology requires much more future work. The joint collaboration will continue to tackle various fundamental and application questions such as a techno-economic analysis and the dynamic behaviourof the working and auxiliary electrodes in the tap water electrolyte.
More information: Xiaoyu Yan et al, A membrane-free flow electrolyzer operating at high current density using earth-abundant catalysts for water splitting, Nature Communications (2021). DOI: 10.1038/s41467-021-24284-5