Credit: Unsplash/CC0 Public Domain
For decades, scientists have been searching for a catalyst that dramatically reduces the cost of fabricating hydrogen fuel cells.
Such an advancement could lead to a green power revolution, with everything from laptops to locomotives running on a fuel whose only byproduct is water.
New research led by the University at Buffalo suggests that scientists are moving closer to that goal.
In a study published Thursday (July 7) in Nature Energy, scientists describe how iron can be combined with nitrogen and carbon to produce a catalyst that is efficient, durable and inexpensive—the three main objectives the U.S. Department of Energy (DOE) has identified for fuel cell research.
"This has been years in the making," says the study's lead author Gang Wu, Ph.D., professor of chemical and biological engineering in the UB School of Engineering and Applied Sciences. "We believe this is a significant breakthrough that will eventually help unleash the tremendous potential of hydrogen fuel cells."
The promise of fuel cells
Fuel cells work like batteries, but they do not run out of power or need recharging, according to DOE. They produce electricity and heat as long as fuel—such as hydrogen—is supplied.
They have long tantalized scientists, environmentalists and others because they have lower or zero emissions compared to combustion engines. And they can be used in a wide range of applications, providing power for vehicles, power plants, buildings and other systems.
But fuel cells are not widely commercialized because, among other things, they require expensive catalysts, which speed up important fuel cell reactions.
The best catalysts have been a family of six precious metals—known as platinum-group metals. While efficient and durable, these metals are incredibly expensive because they are extremely rare. As a result, scientists are seeking less costly alternatives.
Overcoming barriers
One such alternative has been iron-based catalysts. Iron is appealing because it is abundant and inexpensive. But it does not perform as well as platinum, especially because it lacks the durability to withstand the highly corrosive and oxidative environments inside fuel cells.
To overcome this barrier, the research team bonded four nitrogen atoms to the iron. Researchers then embedded the material in a few layers of graphene "with accurate atomic control of local geometric and chemical structures," Wu says.
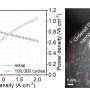
The resulting structure is a vastly improved catalyst. For example, the research team reported the catalyst:
- Is believed to be the most efficient iron-based catalyst produced to date, exceeding the DOE's 2025 target for electric current density.
- Achieved a durability rating that approaches platinum group catalysts.
All this, Wu says, points to the iron-based catalyst's potential to make fuel cells, particularly hydrogen fuel cells, much more affordable for commercial use. Researchers are planning follow-up studies to further improve the catalyst.
More information: Shengwen Liu et al, Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells, Nature Energy (2022). DOI: 10.1038/s41560-022-01062-1
Journal information: Nature Energy
Provided by University at Buffalo