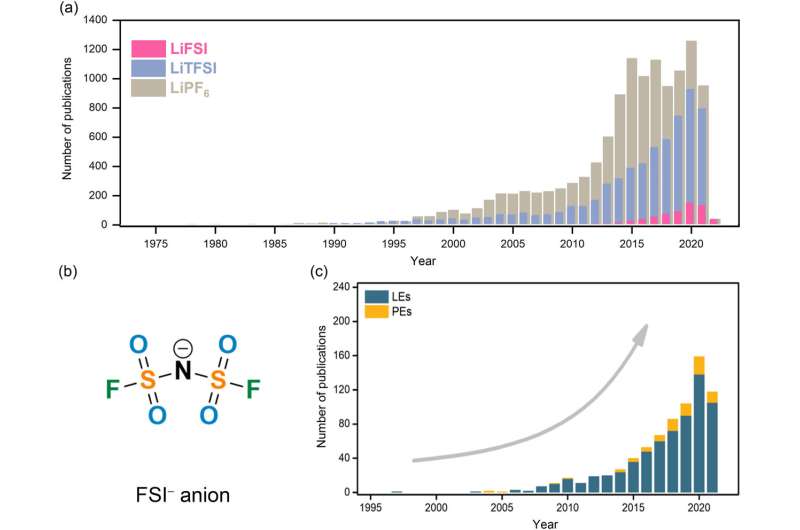
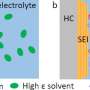
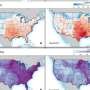
(a) Comparison in the evolution of research interest (i.e., number of publications) of bis(fluorosulfonyl)imide (FSI)-, bis(trifluoromethanesulfonyl)imide (TFSI)-, and hexafluorophosphate (PF6)-based salts in rechargeable lithium batteries. (b) Chemical structure of FSI− anion (c) Evolution of research interest (i.e., number of publications). on the application of FSI− anion in rechargeable lithium batteries. Data are obtained from Scifinder on the January 9, 2022 via structure search of corresponding anions followed by keyword search: "battery" and "lithium." LEs: liquid electrolytes; PEs: polymer electrolytes. Credit: Journal of Power Sources Advances (2022). DOI: 10.1016/j.powera.2022.100088
Researchers worldwide are exploring ways to improve the performance of the rechargeable lithium-ion and lithium metal batteries that power everything from phones and laptops to electric vehicles. In an article published in the open access Journal of Power Sources Advances, researchers in China review new options for the conductive medium at the heart of a battery: the electrolyte.
A battery's electrolyte is a mixture of electrically charged particles (ions) that allows the transfer of electric charge inside the battery. The electrolyte facilitates the reversible movement of ions needed during repeated cycles of battery use and recharging.
The properties of electrolytes are highly dependent on the identity and chemical nature of their negatively charged ions (anions). These support the transfer of positively charged lithium ions through the electrolyte in either direction. Existing electrolytes come with some significant problems, however, including chemical instability, sensitivity to water, volatility and flammability. These issues are driving research into alternative anions that could mitigate the problems and improve battery performance.
One promising alternative being explored for a new generation of batteries is the bis(fluorosulfonyl)imide anion (FSI-), which contains nitrogen, sulfur, oxygen and fluorine atoms.
The paper's authors review the progress of various approaches to making FSI--based electrolytes and assess their performance in a number of prototype lithium-based battery systems. They summarize the advantages of these new electrolytes over the currently dominant systems, highlighting their chemical stability, greater ion mobility, higher conductivity and other favorable chemical features.
The article also addresses a variety of challenges for future research, including developing efficient and environmentally friendly methods for making these electrolytes, and the need for better understanding of their chemical behavior in real battery applications.
The authors expect that research into FSI- ions could also assist work exploring other alternative electrolytes for the batteries of the future. These developments could be important for expanding lithium battery technology into large-scale storage facilities within national power-supply grids.
More information: Ziyu Song et al, Bis(fluorosulfonyl)imide-based electrolyte for rechargeable lithium batteries: A perspective, Journal of Power Sources Advances (2022). DOI: 10.1016/j.powera.2022.100088
Provided by Springer