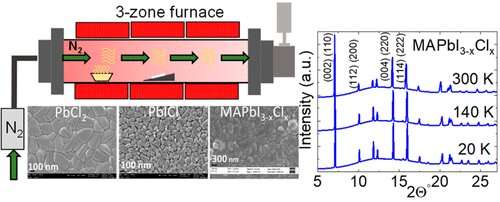
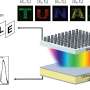
Methylammonium lead iodide (MAPbI3–xClx) thin films were synthesized using chemical vapor deposition. Temperature-dependent grazing incidence synchrotron-based X-ray diffraction measurements confirm that the structure of MAPbI3–xClx, where Cl acts more like a dopant, remains throughout in the tetragonal phase in the temperature range of 20–300 K. These studies are further correlated with temperature-dependent photoluminescence (PL) studies. The PL peak energy monotonically increases with temperature, suggestive of a single-phase behavior. Resistance measurements conducted as a function of temperature show an absence of inflection points, suggesting uniformity in its phase. Between 200 and 325 K, the resistance remains a constant. Credit: ACS Applied Electronic Materials (2022). DOI: 10.1021/acsaelm.2c00449
New materials that can both harvest and emit light offer exciting potential for technologies that range from solar cells to TV and display screens. In a new study, researchers have developed a new way of enhancing the stability and performance of a particular type of these materials, called perovskites. A paper based on the study has been published in ACS Applied Electronic Materials.
Researchers from the University of Missouri, in collaboration with scientists from the University of Western Cape in South Africa and physicists at the U.S. Department of Energy's (DOE) Argonne National Laboratory, have developed a new way to make hybrid perovskites. These are a combination of organic and inorganic semiconducting materials that could form the basis of new solar cells or other electronic devices.
"Organic-inorganic hybrid perovskites have become increasingly attractive to the materials and electronics communities, especially over the past 10 years or so," said MU professor Suchismita (Suchi) Guha, the lead author of the study. "They have become, in some cases, as efficient as silicon-based solar cells. Additionally, they are also much more versatile than silicon and can be used and tuned for a broad array of applications."
Guha and her collaborators improved the methods for making lead halide perovskites. Previous techniques for making these thin-film perovskites required liquid processing using solvents, which rendered the films susceptible to degradation when exposed to air. Additionally, with this prior manufacturing process, one of the molecules would undergo a change to its structure, causing performance limitations in real-world operating conditions.
With the new technique, the researchers were able to prevent the change, holding the affected molecule in a stable structure throughout a large temperature range. Additionally, the new technique rendered the perovskite air stable, making it appropriate for a potential solar cell.
"There have been many studies that have looked at ways to try to improve the stability of hybrid perovskites, including diffusion barriers, additive engineering, and chemically inert electrode optimization, but this is one of the first studies to look at the growth method itself as a way to boost the final performance of the device," Guha said.
To confirm the molecular structure of the perovskite material, Guha and her colleagues, including Argonne physicist Evguenia (Jenia) Karapetrova, used X-ray diffraction measurements at Argonne's Advanced Photon Source (APS), a DOE Office of Science user facility.
"Being able to characterize the perovskite structure at the APS provides a unique window into the possibilities of this functional material," Karapetrova said.
"Preventing the phase change seems to be the key to ensure improved device performance," Guha said. "By maintaining a stable structure throughout the operating temperature window, we show the way to an improved and potentially useful perovskite."
More information: Randy Burns et al, Temperature-Dependent Phase Stable Hybrid Halide Perovskite Films by Chemical Vapor Deposition, ACS Applied Electronic Materials (2022). DOI: 10.1021/acsaelm.2c00449
Provided by Argonne National Laboratory