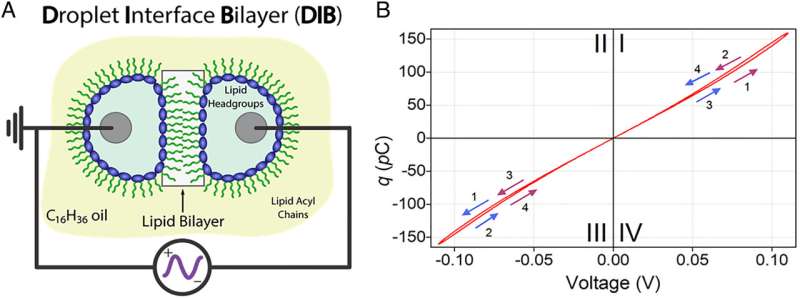
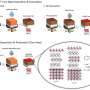
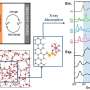
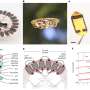
Memcapacitance and DIBs. (A) Schematic of a DIB made from DPhPC, hexadecane (C16H34) and water droplets. The lipid bilayer (boxed region) spontaneously forms when two lipid-coated water droplets suspended in hexadecane from silver/silver chloride (Ag/AgCl) electrodes (black lines and gray circles) are brought into close contact. As the bilayer is being formed, the hexadecane in which the droplets reside is depleted in the bilayer region due to entropic interactions (20–23). The water droplets are fractions of millimeters in diameter, while the lipid bilayer is ~4 nm thick. (B) Charge (the time integral of the current) versus voltage (q-V) in response to a sinusoidal voltage waveform results in a pinched hysteresis loop, characteristic of a memcapacitive system (20, 22). The numbered arrows indicate the directionality of the voltage polarity, from positive to negative (purple arrows) or negative to positive (blue arrows). The frequency of the sinusoidal voltage waveform is set to 0.01 Hz and varied in equal steps of 0.97 µV as follows: 0, 0.12, 0, −0.12, and 0 V (purple arrows) or 0, −0.12, 0, 0.12, and 0 V (blue arrows). It takes 99 s to collect a complete pinched hysteresis loop. Credit: Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2212195119
Ralph Lydic, professor in the UT Department of Psychology, and Dmitry Bolmatov, a research assistant professor in the UT Department of Physics and Astronomy, are part of a UT/ORNL research team studying how bio-inspired materials might inform the design of next-generation computers. Their results, published recently in the Proceedings of the National Academy of Sciences, could have big implications for both edge computing and human health.
Scientists at ORNL and UT discovered an artificial cell membrane is capable of long-term potentiation, or LTP, a hallmark of biological learning and memory. This is the first evidence that a cell membrane alone—without proteins or other biomolecules embedded within it—is capable of LTP that persists for many hours. It is also the first identified nanoscale structure in which memory can be encoded.
"When facilities were shut down as a result of COVID, this led us to pivot away from our usual membrane research," said John Katsaras, a biophysicist in ORNL's Neutron Sciences Directorate specializing in neutron scattering and the study of biological membranes at ORNL. "Together with postdoc Haden Scott, we decided to revisit a system previously studied by Pat Collier and co-workers, this time with an entirely different electrical stimulation protocol that we termed 'training.'"
This eventually led to data that are practically indistinguishable from the LTP signal observed in human brains.
Encoding memory in nanoscale systems has the potential to advance the development of next-generation computing materials and architectures that seek to match the efficiency and flexibility of human cognition—known as neuromorphic computing. While the implications for artificial intelligence may be obvious, brain-like computation will also dramatically alter the energy efficiency and computing capabilities of next-generation devices.
"Memory and logic in the brain are intertwined," said Collier, staff research scientist at the Center for Nanophase Materials Sciences, a DOE Office of Science user facility at ORNL where the research was performed. "But in modern computers, these functions happen in different locations—a bottleneck the brain does not have."
Even today's supercomputers have separate locations for processing and memory. By merging these functions, neuromorphic computers could help keep pace with exponentially growing data sets that are becoming more complex as the Internet of Things, or IoT, and the interconnectivity of devices become commonplace in homes and workspaces. It would also greatly advance edge computing, the ability of a device to do its own logic at the site of data collection, without having to send information to a central server or cloud.
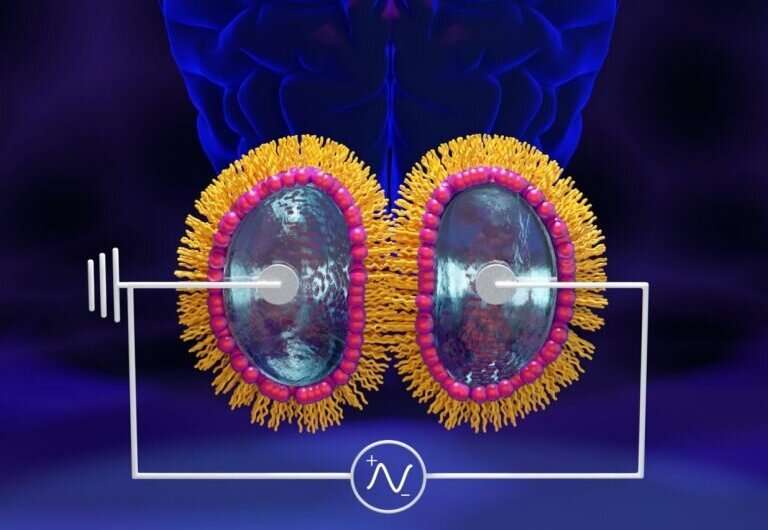
A pure lipid membrane formed using lipid-coated water droplets exhibits long-term potentiation, or LTP, associated with learning and memory, emulating hippocampal LTP observed in the brains of mammals and birds. Credit: Jill Hemman/ORNL, U.S. Dept. of Energy
Also, scientists have not yet identified a nanoscale structure in the brain where memory is stored. Large sections of the brain, such as the hippocampus, are known to store memory, but much remains unknown about where memory is stored in the hippocampus and the molecular mechanisms responsible for it. Importantly, cellular membranes have been overlooked as structures in which information could be encoded, even though lipids, a major component of membranes, make up most of the brain's mass.
The unexpected result of achieving LTP in a pure lipid membrane will initiate a re-examination of where and how memory is stored in a living brain. If neural cell membranes are found to be a critical feature in human memory, this could lead to novel treatments for the more than a billion people worldwide that are living with neurological disorders.
"If neurobiologists can find evidence of this in the brain, it could have dramatic impacts on how we understand dementia and learning," said Katsaras. "Importantly, the membrane can offer a novel therapeutic target for brain diseases that do not respond to drugs targeting proteins."
The nanoscale systems used in this study create an artificial membrane by bringing together two micron-sized lipid-coated water droplets within an oil suspension. At the interface between the two droplets, a lipid bilayer forms that mimics the cell membranes of neuronal synapses in the human brain.
Previous ORNL research showed that this biomembrane system is capable of storing an electric charge, but only for short periods of time. In the new study, the presence of LTP means that there are new avenues for how this soft material system could be used in neuromorphic devices or how it could serve as a model for the construction of solid-state devices with similar features.
"Now that we've begun to define the electrical protocols to induce LTP in lipid bilayer membranes, we are preparing to make two-terminal crossbar architectures in which multiple nanoscale membranes interact, allowing for active logic to be performed, not just passive storage," said Collier. "Right now, we're using single systems; going forward, we need to learn how to wire them together."
In addition to partnering with neurobiologists to explore the biomedical implications of this finding, future neuromorphic computing work on the biomembrane system will involve simulations and use of ORNL's leadership facilities in neutrons and computing.
"What we're seeing are serendipitous discoveries that came from somewhat curiosity-driven research conducted during the pandemic," said Collier. "But it's a significant finding for neuromorphic computing. We don't know exactly how this is going to work, but that's the fun part."
More information: Haden L. Scott et al, Evidence for long-term potentiation in phospholipid membranes, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2212195119
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Tennessee at Knoxville