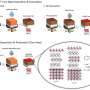
Credit: Carbon (2023). DOI: 10.1016/j.carbon.2023.01.009
A new North Carolina State University study, performed in collaboration with battery testing researchers at the U.S. Department of Energy's Oak Ridge National Laboratory, shows that extremely short pulses from a high-powered laser can cause tiny defects in lithium-ion battery materials—defects that can enhance battery performance.
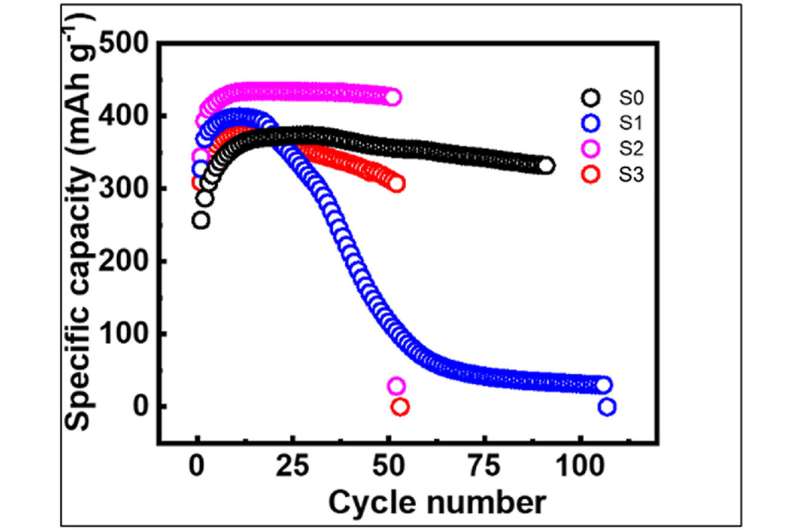
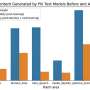
The technique, called nanosecond pulsed laser annealing, lasts for only 100 nanoseconds and is generated by the same type of laser used in modern-day eye surgeries. Researchers tested the technique on graphite, a material widely used in lithium-ion battery anodes, or positive electrodes. They tested the technique in batches of 10 pulses and 80 pulses and compared the differences in current capacity; power is calculated by multiplying voltage by current.
Lithium-ion batteries are widely used in portable electronic devices and electric cars. With further improvements, these batteries could have a major impact on transportation and as storage devices for renewable energy sources like wind and solar.
The study showed a number of interesting results, said Jay Narayan, the John C. Fan Family Distinguished Chair in Materials Science at NC State and corresponding author of a paper describing the work. Narayan pioneered the use of lasers to create and manipulate defects in semiconductors in work spanning more than four decades. The work is published in the journal Carbon.
"Material defects can be a nuisance, but if you engineer them correctly you can make them an advantage," he said. "This technique opens the door, so to speak, for lithium ions, so it enhances the current capacity. Graphite anodes consist of steps and grooves on the surface—creating more steps is like creating more doors for lithium ions to get in and get out, which is beneficial.
"The technique also creates defects called vacancies, which are missing atoms, and that helps provide more sites for lithium ions to come and go, which is related to the current capacity."
Current capacity increased by 20% when the optimal number of pulses was used, which was closer to 10 than to 80 pulses.
The study also showed, though, that too much of a good thing can be a bad thing, as too many defects in the graphite anodes can lead to problems.
"Lithium ion has a positive charge, so if it captures an electron it becomes lithium metal, and you don't want that," Narayan said. "Lithium metal shoots out tiny wire dendrites from the graphite anode and can cause a fire. So you want to make sure that a lithium ion doesn't become a metal."
Narayan said that manufacturers should have the capability to use nanosecond pulse laser annealing when producing both anodes and cathodes, the other electrodes contained in batteries.
"These high-powered lasers exist, and you can treat anodes and cathodes within a microsecond," Narayan said. "The cathodes or anodes are made on a sheet, which makes treatment relatively fast and easy."
Narayan and colleagues at the University of Texas-Austin recently published another paper that used the same laser technique on cathode materials. Published in ACS Applied Materials and Interfaces, that study showed laser treatment enhanced cathode materials.
"Next, we are trying to eliminate the need for using more expensive materials, such as cobalt in battery cathodes, in order to make higher power and longer-lasting batteries," Narayan said.
Roger Narayan, Distinguished Professor of Biomedical Engineering at NC State, co-authored the paper in Carbon along with first author Nayna Khosla, an NC State graduate student. Xiao-Guang Sun and M. Parans Paranthaman from Oak Ridge National Laboratory also co-authored the paper.
More information: Nayna Khosla et al, Microstructure and defect engineering of graphite anodes by pulsed laser annealing for enhanced performance of lithium-ion batteries, Carbon (2023). DOI: 10.1016/j.carbon.2023.01.009
Zehao Cui et al, Laser-Assisted Surface Lithium Fluoride Decoration of a Cobalt-Free High-Voltage Spinel LiNi0.5Mn1.5O4 Cathode for Long-Life Lithium-Ion Batteries, ACS Applied Materials & Interfaces (2022). DOI: 10.1021/acsami.2c18918
Journal information: ACS Applied Materials and Interfaces , Carbon
Provided by North Carolina State University