Proof-of-concept nanogenerator turns CO₂ into sustainable power
University of Queensland researchers have built a generator that absorbs carbon dioxide (CO2) to make electricity.
4 hours ago
1
32
Electronics & Semiconductors

University of Queensland researchers have built a generator that absorbs carbon dioxide (CO2) to make electricity.
4 hours ago
1
32
Energy & Green Tech

A new DECHEMA report "Carbon for Power-to-X—Suitable CO2 sources and integration in PtX value chains" deals with possibilities of capturing and utilizing carbon dioxide for sustainable production routes. Carbon dioxide ...
Apr 15, 2024
0
5
Energy & Green Tech

It's a major contributor to climate change—the way buildings and roads are made with concrete. It's also a problem that's growing as more of the world develops. So the race has been on to find solutions for a material that's ...
Apr 12, 2024
1
64
Energy & Green Tech

For more than two millennia, paper has been a staple of human civilization. But these days, the use of paper is not limited to writing. It is also playing a pivotal role in ushering in a greener future.
Apr 3, 2024
0
35
Electronics & Semiconductors
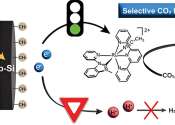
Researchers in the UNC-Chapel Hill Chemistry Department are using semiconductors to harvest and convert the sun's energy into high-energy compounds that have the potential to produce environmentally friendly fuels.
Apr 2, 2024
0
74
Engineering
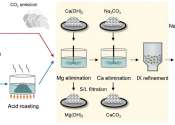
Manufacturers of secondary battery cells (LG Energy Solution, Samsung SDI, and SK) have been insisting on very stringent purity specifications from suppliers of cathode materials to ensure a consistent quality output.
Apr 1, 2024
0
44
Engineering

The construction sector today faces several challenges. Natural sand is fast becoming a scarce resource—we might run out of it by 2050. Carbon dioxide emissions, especially from manufacturing cement or fired clay bricks, ...
Mar 27, 2024
0
3
Engineering
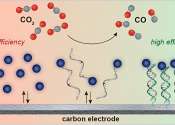
MIT chemical engineers have devised an efficient way to convert carbon dioxide to carbon monoxide, a chemical precursor that can be used to generate useful compounds such as ethanol and other fuels.
Mar 27, 2024
0
175
Energy & Green Tech

Japan announced plans on Wednesday to develop a next-generation passenger jet over the next decade after the last struggling attempt, led by a private company, was scrapped a year ago.
Mar 27, 2024
0
43
Automotive

Hyundai on Wednesday revealed plans to invest more than $50 billion in South Korea by 2026, with a huge chunk dedicated to boosting the development and production of electric vehicles.
Mar 27, 2024
0
5
Carbon dioxide (chemical formula: CO2) is a chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom. It is a gas at standard temperature and pressure and exists in Earth's atmosphere in this state.
Carbon dioxide is used by plants during photosynthesis to make sugars, which may either be consumed in respiration or used as the raw material to produce other organic compounds needed for plant growth and development. It is produced during respiration by plants, and by all animals, fungi and microorganisms that depend either directly or indirectly on plants for food. It is thus a major component of the carbon cycle. Carbon dioxide is generated as a by-product of the combustion of fossil fuels or the burning of vegetable matter, among other chemical processes. Large amounts of carbon dioxide are emitted from volcanoes and other geothermal processes such as hot springs and geysers and by the dissolution of carbonates in crustal rocks.
As of March 2009[update], carbon dioxide in the Earth's atmosphere is at a concentration of 387 ppm by volume. Atmospheric concentrations of carbon dioxide fluctuate slightly with the change of the seasons, driven primarily by seasonal plant growth in the Northern Hemisphere. Concentrations of carbon dioxide fall during the northern spring and summer as plants consume the gas, and rise during the northern autumn and winter as plants go dormant, die and decay. Carbon dioxide is a greenhouse gas as it transmits visible light but absorbs strongly in the infrared and near-infrared.
Carbon dioxide has no liquid state at pressures below 5.1 atmospheres. At 1 atmosphere (near mean sea level pressure), the gas deposits directly to a solid at temperatures below −78 °C and the solid sublimes directly to a gas above −78 °C. In its solid state, carbon dioxide is commonly called dry ice.
CO2 is an acidic oxide: an aqueous solution turns litmus from blue to pink. It is the anhydride of carbonic acid, an acid which is unstable and is known to exist only in aqueous solution.
CO2 is toxic in higher concentrations: 1% (10,000 ppm) will make some people feel drowsy. Concentrations of 7% to 10% cause dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.
This text uses material from Wikipedia, licensed under CC BY-SA