Team develops stable, efficient, anode-free sodium battery
When it comes to batteries, lithium-ion are the best we have as far as energy density and convenience.
May 3, 2021
1
2527
Electronics & Semiconductors

When it comes to batteries, lithium-ion are the best we have as far as energy density and convenience.
May 3, 2021
1
2527
Engineering

Clean energy is the leading solution for climate change. But solar and wind power are inconsistent at producing enough energy for a reliable power grid. Alternatively, lithium-ion batteries can store energy but are a limited ...
Jan 13, 2023
1
68
Energy & Green Tech

LSU researchers are exploring new ways to use the oldest energy source on our planet—sunlight—to create truly green energy on demand. You've already heard of solar cells and solar panels, but David Vinyard, assistant ...
Jan 24, 2022
0
32
Energy & Green Tech
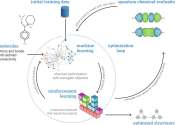
Recent advancements in the development of machine learning and optimization techniques have opened new and exciting possibilities for identifying suitable molecular designs, compounds, and chemical candidates for different ...
Energy & Green Tech

Ammonia has sustained humanity since the early 20th century, but its production leaves a huge carbon footprint. Now researchers have found a way to make it 100 percent renewable.
Jan 20, 2021
1
204
Energy & Green Tech

Batteries made from an electrically conductive mixture the consistency of molasses could help solve a critical piece of the decarbonization puzzle. An interdisciplinary team from MIT has found that an electrochemical technology ...
Nov 30, 2021
2
544
Energy & Green Tech

The 100 MW Dalian Flow Battery Energy Storage Peak-shaving Power Station, with the largest power and capacity in the world so far, was connected to the grid in Dalian, China, on September 29, and it will be put into operation ...
Sep 29, 2022
0
42
Energy & Green Tech

In recent years, researchers have been exploring the potential of a wide range of new battery technologies, including so-called redox flow batteries. Redox flow batteries, also known as flow batteries, are battery cells that ...
Energy & Green Tech

In any form of energy conversion—even with something as green as solar panels—extra heat is generated. But with up to 72 percent of it left unused, there's also great potential to harvest electricity from that waste.
Sep 21, 2021
0
10
Energy & Green Tech

Energy storage company Highview Power has announced its intention to build a cryogenic energy storage facility in the north of England—a first for the U.K. The project calls for converting a decommissioned thermal power ...
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.
The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the "fundamental equations of Gibbs" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.
This text uses material from Wikipedia, licensed under CC BY-SA