First battery prototype using hemoglobin developed
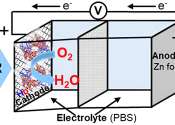
A team with the Chemical Institute for Energy and the Environment (IQUEMA) at the University of Cordoba has come up with a battery that uses hemoglobin as an electrochemical reaction facilitator, functioning for around 20–30 ...
Jan 9, 2024
0
46