The biggest barrier to a vibrant second-hand electric vehicle market? Price
News policies and broader subsidies are needed to help lower-income buyers afford used electric vehicles, according to a Rutgers study.
Apr 19, 2024
0
17
Consumer & Gadgets

News policies and broader subsidies are needed to help lower-income buyers afford used electric vehicles, according to a Rutgers study.
Apr 19, 2024
0
17
Engineering

A team of engineering students at the University of Bath have achieved a world first by becoming the first undergraduates to build and successfully run a hydrogen-fueled engine.
Apr 17, 2024
0
92
Energy & Green Tech

Nissan expects to mass produce electric vehicles powered by advanced next-generation batteries by early 2029, the company said Tuesday during a media tour of an unfinished pilot plant.
Apr 16, 2024
0
20
Automotive

British luxury carmaker Aston Martin Lagonda will continue to produce traditional combustion-engine vehicles for as long as legally possible, its boss told UK media this week.
Apr 11, 2024
0
4
Energy & Green Tech

Steam locomotives clattering along railway tracks. Paddle steamers churning down the Murray. Dreadnought battleships powered by steam engines.
Mar 18, 2024
0
9
Energy & Green Tech

While the transportation sector has witnessed a dramatic shift toward electric vehicles (EVs), the idea of using hydrogen as a clean and efficient fuel for transportation has been explored for many decades. These vehicles ...
Mar 5, 2024
0
60
Automotive

Thousands of cars from China's BYD rolled off a ship in the German port of Bremerhaven on Monday, as the world's biggest electric carmaker brought its challenge directly to Europe's auto making powerhouse.
Feb 26, 2024
0
4
Automotive

Last fall's contentious United Auto Workers' strike changed Ford's relationship with the union to the point where it will "think carefully" about where it builds future vehicles, Ford's top executive said Thursday.
Feb 15, 2024
0
19
Automotive

China's global dominance in electric cars helped it overtake Japan as the world's biggest vehicle exporter last year, official data confirmed Wednesday.
Jan 31, 2024
0
1
Energy & Green Tech
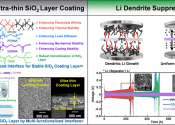
Lithium-ion batteries are a widely used class of rechargeable batteries in today's world. One of the processes that can hamper the functioning of these batteries is an internal short circuit caused by direct contact between ...
Jan 25, 2024
0
38
Combustion (English pronunciation: /kəmˈbʌs.tʃən /) or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame. Fuels of interest often include organic compounds (especially hydrocarbons) in the gas, liquid or solid phase.
In a complete combustion reaction, a compound reacts with an oxidizing element, such as oxygen or fluorine, and the products are compounds of each element in the fuel with the oxidizing element. For example:
A simple example can be seen in the combustion of hydrogen and oxygen, which is a commonly used reaction in rocket engines:
The result is water vapor.
Complete combustion is almost impossible to achieve. In reality, as actual combustion reactions come to equilibrium, a wide variety of major and minor species will be present such as carbon monoxide and pure carbon (soot or ash). Additionally, any combustion in atmospheric air, which is 78% nitrogen, will also create several forms of nitrogen oxides.
This text uses material from Wikipedia, licensed under CC BY-SA