Plasma treatment enhances electrode material for fuel cells in industry, homes and vehicles
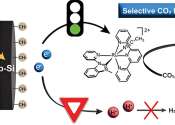
Researchers from Skoltech and their colleagues have improved the properties of a carbon-based electrode material by exposing it to air plasma. Such treatment turned out to enhance electrode performance, which is the limiting ...
Apr 22, 2024
0
40