Retro-reflectors could help future cities keep their cool
Engineers at Princeton University have quantified the cooling benefits of a simple solution for beating urban heat: reflecting solar radiation back from whence it came.
Apr 17, 2024
0
50
Engineering
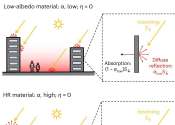
Engineers at Princeton University have quantified the cooling benefits of a simple solution for beating urban heat: reflecting solar radiation back from whence it came.
Apr 17, 2024
0
50
Engineering

Most people do not give the U.S. electric grid a second thought—we flip a switch, and the lights come on. Behind the scenes are thousands of power plants and utilities linked by millions of miles of transmission lines. ...
Apr 16, 2024
0
57
Engineering

Controlling heat flow is key to enhancing performance in a wide variety of systems. In electronic devices, such as mobile phones or any type of processor, overheating decreases their performance and reduces their lifetime. ...
Apr 11, 2024
0
54
Engineering

The Compression-Absorption Cascade Refrigeration Cycle (CACRC) system, merging Vapor-Compression Refrigeration (VCR) with Absorption Refrigeration Cycle (ARC), presents a promising answer to the pressing energy demands and ...
Apr 8, 2024
0
3
Engineering

There is room for just one small bottle in the world's first refrigerator that is cooled with artificial muscles made of nitinol, a nickel-titanium alloy. But the mini-prototype that the team led by professors Stefan Seelecke ...
Apr 2, 2024
0
39
Energy & Green Tech

There may be few better real-life laboratories in the United States than Cordova, Alaska, to evaluate the challenges and benefits of transitioning to new, cleaner methods of home heating in a colder climate.
Apr 2, 2024
0
33
Electronics & Semiconductors

A new electrical power converter design achieves a much higher efficiency at lower cost and maintenance than before. The direct current voltage boost converter developed by Kobe University is poised to be a significant contribution ...
Apr 1, 2024
0
134
Energy & Green Tech

Anyone who has ever hot-footed it barefoot across the beach on a sunny day walks away with a greater understanding of just how much heat sand can retain. That ability is expected to play a vital role in the future, as technology ...
Mar 29, 2024
0
13
Engineering

Compared with other cutting methods, EV-chiseling could generate metallic microstructures with ultra-high aspect ratio, and the cutting chip could be directly transformed into unique microstructures.
Mar 28, 2024
0
6
Energy & Green Tech

A Washington State University-led study found that widespread, extreme temperature events are often accompanied by greater solar radiation and higher wind speeds that could be captured by solar panels and wind turbines. The ...
Mar 27, 2024
0
54
In physics and thermodynamics, heat is the process of energy transfer from one body or system to another due to a difference in temperature. In thermodynamics, the quantity TdS is used as a representative measure of the (inexact) heat differential δQ, which is the absolute temperature of an object multiplied by the differential quantity of a system's entropy measured at the boundary of the object.
A related term is thermal energy, loosely defined as the energy of a body that increases with its temperature. Heat is also loosely referred to as thermal energy, although many definitions require this thermal energy to actually be in the process of movement between one body and another to be technically called heat (otherwise, many sources prefer to continue to refer to the static quantity as "thermal energy"). Heat is also known as "Energy".
Energy transfer by heat can occur between objects by radiation, conduction and convection. Temperature is used as a measure of the internal energy or enthalpy, that is the level of elementary motion giving rise to heat transfer. Energy can only be transferred by heat between objects - or areas within an object - with different temperatures (as given by the zeroth law of thermodynamics). This transfer happens spontaneously only in the direction of the colder body (as per the second law of thermodynamics). The transfer of energy by heat from one object to another object with an equal or higher temperature can happen only with the aid of a heat pump, which does work.
This text uses material from Wikipedia, licensed under CC BY-SA