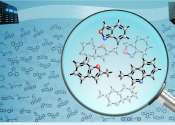
New iron catalyst could make hydrogen fuel cells affordable
For decades, scientists have been searching for a catalyst that dramatically reduces the cost of fabricating hydrogen fuel cells.
Jul 7, 2022
1
11820
Energy & Green Tech

For decades, scientists have been searching for a catalyst that dramatically reduces the cost of fabricating hydrogen fuel cells.
Jul 7, 2022
1
11820
Engineering

A new kind of solar panel, developed at the University of Michigan, has achieved 9% efficiency in converting water into hydrogen and oxygen—mimicking a crucial step in natural photosynthesis. Outdoors, it represents a major ...
Jan 4, 2023
2
326
Energy & Green Tech

Hydrogen production takes place using natural gas as the raw material, combined with a very special ceramic membrane. Both hydrogen production and CO2 capture are achieved in a single step, which makes the method highly energy ...
Jul 28, 2022
1
185
Energy & Green Tech

Researchers at Princeton and Rice universities have combined iron, copper, and a simple LED light to demonstrate a low-cost technique that could be key to distributing hydrogen, a fuel that packs high amounts of energy with ...
Nov 25, 2022
0
134
Energy & Green Tech

LSU researchers are exploring new ways to use the oldest energy source on our planet—sunlight—to create truly green energy on demand. You've already heard of solar cells and solar panels, but David Vinyard, assistant ...
Jan 24, 2022
0
32
Energy & Green Tech

In the continued effort to move humanity away from fossil fuels and towards more environmentally friendly energy sources, researchers in Japan have developed a new material capable storing hydrogen energy in a more efficient ...
Oct 26, 2023
0
33
Engineering

Using solar energy to inexpensively harvest hydrogen from water could help replace carbon-based fuel sources and shrink the world's carbon footprint. However, finding materials that could boost hydrogen production so that ...
Jun 17, 2021
1
18
Engineering

Researchers at Kyoto University's Institute for Cell-Material Sciences (iCeMS) have developed a new approach to speed up hydrogen atoms moving through a crystal lattice structure at lower temperatures. They reported their ...
Jun 2, 2021
0
16
Energy & Green Tech

Engineers at the University of Illinois Chicago have built a machine that captures carbon from flue gas and converts it to ethylene.
Dec 12, 2022
0
141
Energy & Green Tech
In a computational study leveraging artificial intelligence (AI), scientists at the U.S. Department of Energy's (DOE) Argonne National Laboratory have assessed 160 billion molecules, a number exceeding the people born in ...
Jan 10, 2024
0
122
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force. The most abundant isotope, hydrogen-1, protium, or light hydrogen, contains no neutrons; other isotopes contain one or more neutrons. This article primarily concerns hydrogen-1.
The hydrogen atom has special significance in quantum mechanics and quantum field theory as a simple two-body problem physical system which has yielded many simple analytical solutions in closed-form.
In 1914, Niels Bohr obtained the spectral frequencies of the hydrogen atom after making a number of simplifying assumptions. These assumptions, the cornerstones of the Bohr model, were not fully correct but did yield the correct energy answers. Bohr's results for the frequencies and underlying energy values were confirmed by the full quantum-mechanical analysis which uses the Schrödinger equation, as was shown in 1925/26. The solution to the Schrödinger equation for hydrogen is analytical. From this, the hydrogen energy levels and thus the frequencies of the hydrogen spectral lines can be calculated. The solution of the Schrödinger equation goes much further than the Bohr model however, because it also yields the shape of the electron's wave function ("orbital") for the various possible quantum-mechanical states, thus explaining the anisotropic character of atomic bonds.
The Schrödinger equation also applies to more complicated atoms and molecules. However, in most such cases the solution is not analytical and either computer calculations are necessary or simplifying assumptions must be made.
This text uses material from Wikipedia, licensed under CC BY-SA