New lithium plant inaugurated in Argentina
French mining group Eramet and China's Tsingshan on Wednesday inaugurated a lithium production plant in Argentina to supply the booming electric car industry.
Jul 3, 2024
0
8
Energy & Green Tech

French mining group Eramet and China's Tsingshan on Wednesday inaugurated a lithium production plant in Argentina to supply the booming electric car industry.
Jul 3, 2024
0
8
Engineering

The performance and reliability of machines and devices reduce as they age. Currently, inaccurate degradation characterization leads to over- and under-maintenance, both of which have dire consequences on society. Hence, ...
Jul 3, 2024
0
1
Consumer & Gadgets

More Australians than ever are riding electric bikes—a fact you may have noticed on the streets of our cities and towns.
Jul 3, 2024
0
5
Energy & Green Tech

UChicago Pritzker Molecular Engineering Prof. Y. Shirley Meng's Laboratory for Energy Storage and Conversion has created the world's first anode-free sodium solid-state battery.
Jul 3, 2024
0
71
Energy & Green Tech

Researchers from Hunan University have designed a layered oxide cathode for rechargeable lithium-ion batteries that achieves fast-charging performance, long life, and high safety using only an ultra-low amount of cobalt. ...
Jul 2, 2024
0
5
Energy & Green Tech
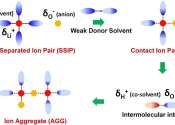
Electric vehicles, large-scale energy storage, polar research and deep space exploration all have placed higher demands on the energy density and low-temperature performance of energy storage batteries. In recent years, lithium ...
Jul 2, 2024
0
35
Energy & Green Tech

A technology has been developed to replace the active material in large-capacity ESS "redox flow batteries" with a more affordable substance.
Jul 2, 2024
0
64
Engineering

It is the battery in your electric car that determines how far you can drive on one charge and how quickly you can re-charge. However, the lithium-ion battery, the most widely used electric car battery today, has its limitations—in ...
Jun 26, 2024
0
25
Energy & Green Tech

With goals to limit CO2 emissions, many countries have set targets to phase out internal combustion vehicles in favor of electric vehicles (EVs). Japan has set a target for 20%–30% of all car sales to be battery electric ...
Jun 25, 2024
0
0
Energy & Green Tech
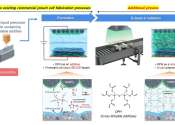
Professor Soojin Park, Seoha Nam, a Ph.D. candidate, and Dr. Hye Bin Son from the Department of Chemistry at Pohang University of Science and Technology (POSTECH) have achieved a breakthrough in creating a gel electrolyte-based ...
Jun 24, 2024
0
51
Lithium (pronounced /ˈlɪθiəm/) is the chemical element with atomic number 3, and is represented by the symbol Li. It is a soft alkali metal with a silver-white color. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals lithium is highly reactive, corroding quickly in moist air to form a black tarnish. For this reason lithium metal is typically stored under the cover of oil. When cut open lithium exhibits a metallic luster, but contact with oxygen quickly turns it back to a dull silvery gray color. Lithium in its elemental state is highly flammable.
According to theory, lithium was one of the few elements synthesized in the Big Bang. Since its current estimated abundance in the universe is vastly less than that predicted by theory; the processes by which new lithium is created and destroyed, and the true value of its abundance, continue to be active matters of study in astronomy. The nuclei of lithium are relatively fragile: the two stable lithium isotopes found in nature have lower binding energies per nucleon than any other stable compound nuclides, save for the exotic and rare deuterium, and 3He. Though very light in atomic weight, lithium is less common in the solar system than 25 of the first 32 chemical elements.
Due to its high reactivity it only appears naturally in the form of compounds. Lithium occurs in a number of pegmatitic minerals, but is also commonly obtained from brines and clays. On a commercial scale, lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride.
Trace amounts of lithium are present in the oceans and in some organisms, though the element serves no apparent vital biological function in humans. However, the lithium ion Li+ administered as any of several lithium salts has proved to be useful as a mood stabilizing drug due to neurological effects of the ion in the human body. Lithium and its compounds have several industrial applications, including heat-resistant glass and ceramics, high strength-to-weight alloys used in aircraft, and lithium batteries. Lithium also has important links to nuclear physics. The transmutation of lithium atoms to tritium was the first man-made form of a nuclear fusion reaction, and lithium deuteride serves as a fusion fuel in staged thermonuclear weapons.
This text uses material from Wikipedia, licensed under CC BY-SA