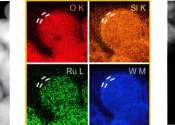
Oxygen (pronounced /ˈɒksɨdʒɨn/, from the Greek roots ὀξύς (oxys) (acid, literally "sharp", from the taste of acids) and -γενής (-genēs) (producer, literally begetter) is the element with atomic number 8 and represented by the symbol O. It is a member of the chalcogen group on the periodic table, and is a highly reactive nonmetallic period 2 element that readily forms compounds (notably oxides) with almost all other elements. At standard temperature and pressure two atoms of the element bind to form dioxygen, a colorless, odorless, tasteless diatomic gas with the formula O2. Oxygen is the third most abundant element in the universe by mass after hydrogen and helium and the most abundant element by mass in the Earth's crust. Diatomic oxygen gas constitutes 20.9% of the volume of air.
All major classes of structural molecules in living organisms, such as proteins, carbohydrates, and fats, contain oxygen, as do the major inorganic compounds that comprise animal shells, teeth, and bone. Oxygen in the form of O2 is produced from water by cyanobacteria, algae and plants during photosynthesis and is used in cellular respiration for all complex life. Oxygen is toxic to obligately anaerobic organisms, which were the dominant form of early life on Earth until O2 began to accumulate in the atmosphere 2.5 billion years ago. Another form (allotrope) of oxygen, ozone (O3), helps protect the biosphere from ultraviolet radiation with the high-altitude ozone layer, but is a pollutant near the surface where it is a by-product of smog. At even higher low earth orbit altitudes monatomic oxygen (O1) is a significant presence and a cause of erosion for spacecraft.
Oxygen was independently discovered by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774, but Priestley is often given priority because his publication came out in print first. The name oxygen was coined in 1777 by Antoine Lavoisier, whose experiments with oxygen helped to discredit the then-popular phlogiston theory of combustion and corrosion. Oxygen is produced industrially by fractional distillation of liquefied air, use of zeolites to remove carbon dioxide and nitrogen from air, electrolysis of water and other means. Uses of oxygen include the production of steel, plastics and textiles; rocket propellant; oxygen therapy; and life support in aircraft, submarines, spaceflight and diving.