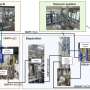
Graphical abstract. Credit: Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.147862
Water electrolysis using renewable energy sources has emerged as a promising clean method for hydrogen production. However, its extensive freshwater consumption poses limitations to regions with abundant water resources. Therefore, it is imperative to develop a new technology for water electrolysis that can directly harness the abundant supply of seawater.
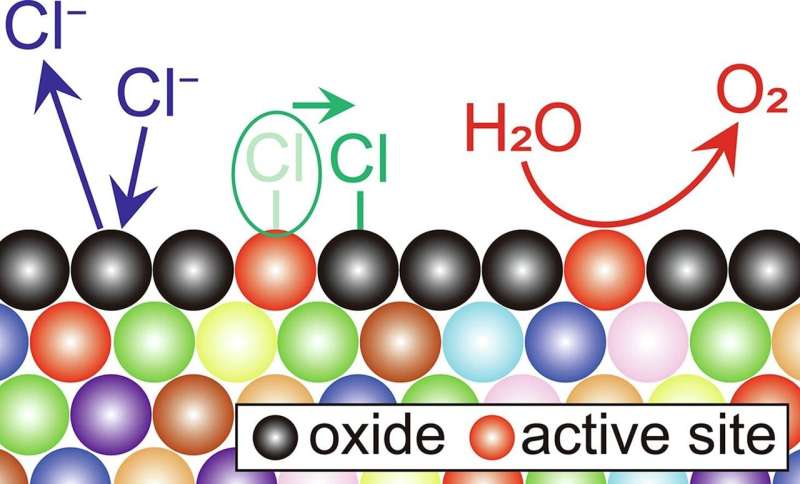
During seawater electrolysis, the anode reaction generates oxygen from water, chlorine gas, and hypochlorous acid from chloride ions. Precious metal electrodes, such as platinum oxide, ruthenium oxide, and iridium oxide, which are unaffected by chlorine, are widely used as anode electrodes.
Although precious metals are undesirable as electrodes for widespread seawater electrolysis technology, non-noble metals, which are highly reactive with chloride ions, cannot be employed for durable anodes.
A research group has developed a multi-elemental alloy electrode composed of nine non-noble metal elements and conducted an accelerated degradation test, consisting of turning the power supply on and off, which mainly caused degradation during the operation of the water electrolysis system. The results suggest sustained anode performances for over a decade when powered by solar energy. Their findings have been published in Chemical Engineering Journal.
The anode made of this alloy requires higher voltages than that of the precious metal, such as iridium oxide. However, this anode offers direct seawater electrolysis without using fresh water. This innovation is expected to transcend geographical restrictions owing to the availability of fresh water, thereby promoting hydrogen production in regions abundant with renewable energy, such as coastal desert areas.
More information: Fumiya Shiokawa et al, Durable high-entropy non-noble metal anodes for neutral seawater electrolysis, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.147862
Journal information: Chemical Engineering Journal
Provided by University of Tsukuba