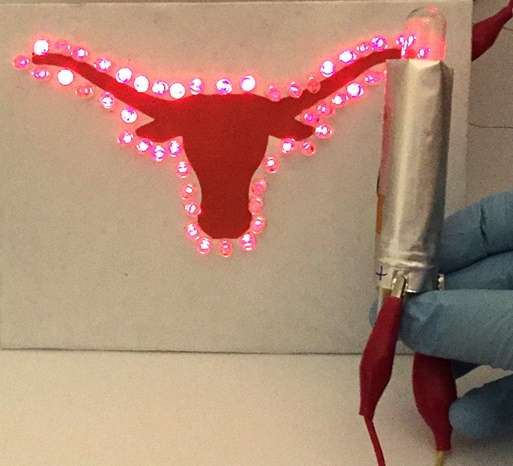
The rolled sodium-ion full-cell battery is shown powering an array of red LEDs in the outline of a longhorn, the logo of the University of Texas at Austin. Credit: Hongsen Li et al. ©2016 The Royal Society of Chemistry
(TechXplore)—Most of the sodium-ion batteries that have been developed so far have been half-cell batteries, meaning that the anode is made of a standard sodium metal. However, this standard sodium metal becomes highly active when exposed to oxygen or moisture, creating a safety hazard. For this reason, researchers have been exploring sodium-ion batteries in a full cell format, in which the anode is made of an alternative material.
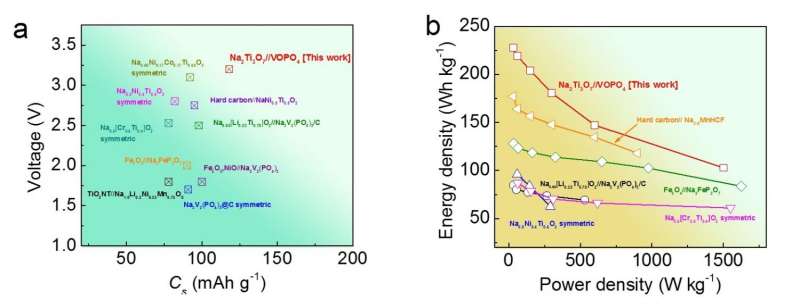
In a new study, researchers have designed and fabricated a sodium-ion full-cell battery that uses sodium titanium oxide nanotubes as the anode material. In addition to greatly reducing the safety risks compared to sodium-ion half-cell batteries, the new battery can store nearly the same amount of energy in a given volume as today's state-of-the-art lithium-ion batteries. Although the new battery's energy density (220 Wh/kg by itself, or an estimated 130 Wh/kg when fully assembled) is not as high as that of the best sodium-ion half-cells, it is the highest achieved so far for sodium-ion full-cell batteries. A high energy density ultimately translates to longer battery lifetimes and—when used in electric vehicles—longer driving ranges.
The researchers, led by Guihua Yu at The University of Texas at Austin, have published a paper on the new high-energy sodium-ion full-cell battery in a recent issue of Energy & Environmental Science.
Over the past several years, researchers have been investigating alternatives to lithium-ion batteries because of the high cost and difficulty in obtaining lithium, most of which is located in politically sensitive or remote areas. Sodium is particularly appealing because of its widespread abundance, making it inexpensive to obtain in large quantities.
However, designing a sodium-ion battery is more difficult than designing a lithium one because sodium ions are larger than lithium ions. The larger size makes it more difficult to rapidly insert and extract sodium ions into and out of the electrodes, and this difficulty places stringent requirements on the electrode materials.
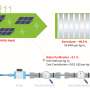
The sodium-ion full cell reported here is one of the top-performing sodium-ion full batteries in terms of several factors, including voltage, capacity, energy density, and power density. Credit: Hongsen Li et al. ©2016 The Royal Society of Chemistry
By fabricating electrodes out of nanostructured materials with layered crystal structures (nanotubes for the anode material and ultrathin nanosheets for the cathode material), the researchers here demonstrated a battery that addresses many performance and safety criteria. These factors include the rate of sodium ion insertion/extraction (or voltage), as well as the rate capability (how quickly the battery can charge/discharge).
Overall, the nanostructured electrode materials used here have achieved what the researchers believe to be the best combination yet of battery characteristics: along with the high energy density mentioned above, the battery also exhibits a high operating voltage (about 3V), large reversible capacity, high rate capability, thermal stability over a wide temperature range, and high capacity retention (over 92% after 100 cycles).
With these characteristics, the researchers expect that the new sodium-ion full-cell battery will be a promising candidate for future large-scale energy storage, such as storing energy generated by wind and solar technologies, as well as electric vehicles.
"Practical applications of sodium-ion full batteries have been hindered by many limitations, such as low working potential, large capacity decay during cycles (short cycle life), and low safety," Yu told Phys.org. "In the next stage, we are aiming to solve the aforementioned issues by continuing to search for more promising electrode materials with a much longer cycle life (from a few hundred to thousands of cycles), as well as higher energy and power densities. In addition, replacing the high volatile and flammable organic electrolytes with solid-state electrolytes or quasi-solid-state electrolytes may provide another promising direction to build long-term safer sodium-ion batteries."
More information: Hongsen Li et al. "An advanced high-energy sodium ion full battery based on nanostructured Na2Ti3O7/VOPO4 layered materials." Energy & Environmental Science. DOI: 10.1039/c6ee00794e
Journal information: Energy & Environmental Science
© 2016 TechXplore