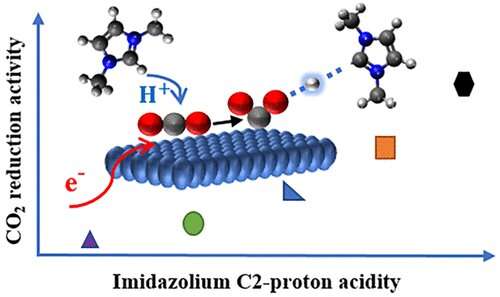
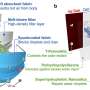
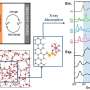
Graphical abstract. Credit: ACS Energy Letters (2022). DOI: 10.1021/acsenergylett.2c01372
Researchers from the University of Twente, in collaboration with Shell, developed a new mechanism that makes the conversion of carbon dioxide into carbon monoxide, which is an essential feedstock in the production of chemicals.
Within this project under the umbrella of the Advanced Research Center Chemical Building Blocks Consortium (ARC CBBC), the researchers published their findings in the journal ACS Energy Letters. Their publication was also selected for the cover images of the same journal. UT Ph.D. student and lead author Sobhan Neyrizi states, "With the novel molecules designed in our research, we could innovate a new pathway for CO2 conversion."
The emission of carbon dioxide is considered one of the major causes of global warming. One possible solution is the electrochemical conversion of carbon dioxide into more useful molecules such as carbon monoxide, formic acid and hydrocarbons, which are important intermediates in fuel and chemical production. However, the energy demand of the reaction is still too high.
New pathway
The new molecules that Sobhan Neyrizi and his fellow researchers have developed can assist the electrochemical conversion of carbon dioxide.
"Our molecules act as co-catalysts that reduce the energy demands of the reaction to a great extent," says Neyrizi. The researchers argue they can use these molecules to innovate a new pathway for the reaction. "We could also propose design principles for the development of more efficient molecules in the future."
100% efficiency
In electrochemistry, electrons are used as a cheap energy source. But transferring electrons to carbon dioxide—which is the key step needed for the conversion—demands too much energy. The energy needed can be drastically reduced by simultaneously transferring protons and electrons to carbon dioxide molecules.
The designed co-catalysts make this simultaneous transfer possible on a surface of gold. "We could reach 100% efficiency for the conversion, which means that all electrons we put into the reaction get used," explains Neyrizi.
Credit: University of Twente
More information: Sobhan Neyrizi et al, What It Takes for Imidazolium Cations to Promote Electrochemical Reduction of CO2, ACS Energy Letters (2022). DOI: 10.1021/acsenergylett.2c01372
Journal information: ACS Energy Letters
Provided by University of Twente