Splitting water using bismuth vanadate
The photocatalytic water splitting process holds appeal for scientists as a solution to both energy and environmental problems. In this process, water is split into oxygen and hydrogen using light energy and a catalyst. As the problem of global warming has increased, researchers have looked to hydrogen, a clean-burning fuel, as a renewable energy solution. With water being such an inexpensive resource, tremendous effort has been dedicated to this promising research field during the past decades. But scientists have only been able to discover a few photocatalysts with both high efficiency and excellent stability. So the photocatalytic water splitting technology still has a long way to go until practical application is possible.
A research team from Xi'an Jiaotong University in China has achieved promising results using an inorganic compound called bismuth vanadate (BiVO4) crystals as a photocatalyst to achieve efficient photocatalytic water splitting. Their work shows the close relationship between the surface properties of the BiVO4 and the photocatalytic activity achieved. The team's findings are published in the journal Nano Research.
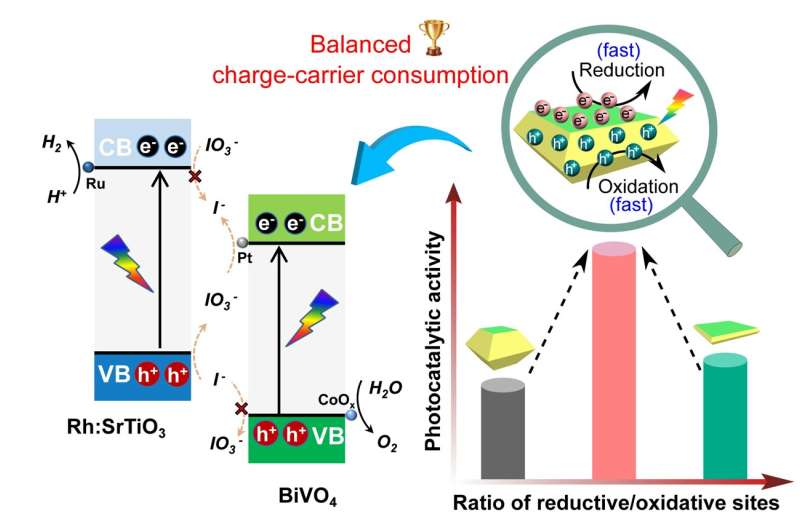
For the water splitting process to be efficient, separation of the electron-hole pair and their consumption by water oxidation or water reduction reactions taking place on the surface is essential. The electron-hole is a charge carrier responsible for creating electric current in semiconducting materials. Charge-carrier refers to a particle that moves freely within a material and carries an electric charge.
In recent years, scientists have achieved excellent performance by exposing specific facets as enriched reaction sites on the photocatalysts. Researchers have discovered that titanium dioxide and strontium titanate, with their exposed facets, offer excellent performance. This knowledge gave scientists clues that an efficient photocatalytic process could be achieved by tuning the surface of a photocatalyst with different functions.
In further research, scientists reported that BiVO4 nanosheets with exposed facets exhibited excellent performance for water oxidation. Research suggested that if the BiVO4 facets were enlarged, superior photocatalytic activity for water oxidation could be achieved.
The Xi'an Jiaotong University research team focused their attention on BiVO4 as a model photocatalyst. They studied the crucial role of surface charge-carrier consumption on the water splitting reactions. The team fabricated BiVO4 single crystals with a tailored ratio of facets for the reductive sites and the oxidative sites. They used a simple controlled hydrothermal method to synthesize the BiVO4 crystal. Through this process, they demonstrated that efficient photocatalytic water oxidation could be achieved through balanced surface charge-carrier consumption that is based on a medium ratio of the reductive sites and the oxidative sites.
Using BiVO4 alone as a typical photocatalyst for water oxidation does not achieve overall water splitting. So the researchers continued their study, constructing a Z-scheme system, where two different photocatalysts are combined. Using the BiVO4 with appropriate cocatalysts, the team achieved efficient and stable photocatalytic overall water splitting.
"Superior photocatalytic water oxidation is obtained from BiVO4 decahedrons with a medium ratio between reductive and oxidative sites, which is ascribed to the as-achieved balanced surficial charge-carrier consumption," said Shaohua Shen, a professor in the International Research Center for Renewable Energy at Xi'an Jiaotong University. "In addition, efficient and stable photocatalytic overall water splitting is achieved by adopting the as-synthesized BiVO4 decahedrons with suitable cocatalyst modification," said Shen.
"Looking ahead, this work provides both guidance on the fabrication of nano/micro-material with controllable surficial morphology and insightful investigations of the corresponding photocatalytic redox reaction," said Shen.
More information: Xiangjiu Guan et al, Photocatalytic water splitting on BiVO4: Balanced charge-carrier consumption and selective redox reaction, Nano Research (2022). DOI: 10.1007/s12274-022-4758-8