December 1, 2022 feature
An amorphous high-capacity iron fluorosulfate cathode
Chemists and engineers have been trying to create increasingly efficient, affordable and stable battery technologies to power a wide range of electronic devices. To do this, they have been leveraging the multi-redox reactions of materials that are highly abundant on Earth, such as iron and manganese, which could help to lower battery fabrication costs.
A team of researchers at Seoul National University has identified a-LiFeSO4F, an amorphous iron fluorisulfate electrode that could be used to develop more affordable high-capacity batteries. This electrode, presented in a paper in Nature Energy, specifically serves as a cathode (i.e., the positively charged electrode in a battery cell, through which electrons enter electronic devices).
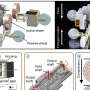
The iron fluorisulfate cathode they created was inspired by a nanocomposite material that they had introduced in one of their previous works, where they also elucidated its underpinning redox mechanism and surface conversion reaction. This material is composed of nanosized lithium and a transition metal compound.
"Previously, we were studying the strategies for obtaining additional capacities from our nanocomposite cathode material," Kisuk Kang, one of the researchers who carried out the study, told TechXplore. "As the redox mechanism of the material is similar to a conversion reaction and is typically synthesized by the high energy ball milling, we were able to attain a better understanding of the conversion reaction and mechanochemical synthesis."
The team's new study is based on the observations and findings collected in their previous research. Specifically, their past findings inspired them to explore the possibility that their nanocomposite cathode material's conversion reaction, combined with an intercalation capability, would allow them to unlock the unique capacity of the transition metal oxides within it.
"During the development of this concept, we pondered on how to effectively enhance the reversibility of an intercalation/conversion dual-type electrode material," Kang explained. "We came to the conclusion that an amorphous structure could help to achieve this, considering the nature of the conversion reaction."
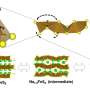
The cathode realized by Kang and his colleagues is made of LiFeSO4F, but with an amorphous (i.e., not crystalline) structure. This material could be easily synthesized using mechanochemical processes, specifically through the high-energy ball milling of lithium fluoride (LiF) and iron sulfate (FeSO4).
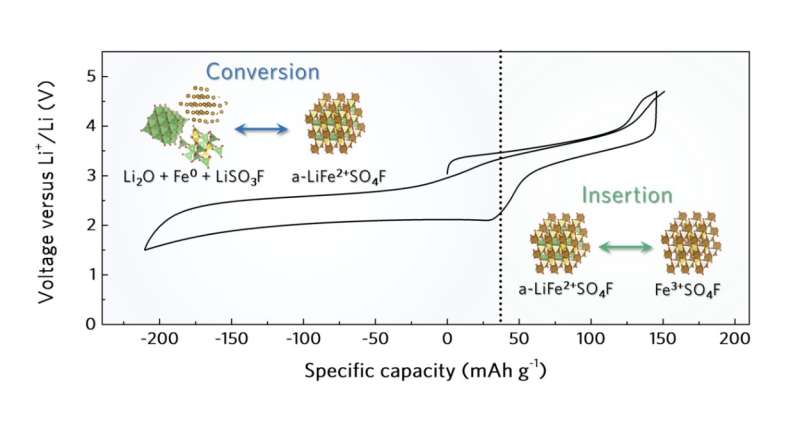
A remarkable advantage of the researchers' cathode is that it supports the reversible insertion and extraction of lithium ions through two processes known as intercalation and conversion. This significantly increases its cumulative capacity, which could in turn improve a battery's life and performance.
"Our study unveiled the novel role of amorphous structure in enabling the reversibility of dual-type intercalation/conversion electrode material," Kang said. "The reversible and simultaneous utilization of intercalation and conversion reactions in the amorphous structure is broadly applicable to other transition metal compounds and thus enlarges the candidate group of high-energy density cathode material."
In the future, the cathode introduced by this team of researchers could be used to create high-capacity and low-cost battery technologies. In addition, its underlying chemical processes might also be replicated using other transition metal compounds, which would open new possibilities for the creation of highly performing cathodes based on Earth-abundant materials.
"Since we verified our concept, we have been exploring a more expansive compositional space to identify other possible cathode materials," Kang added. "Moreover, we are studying the general governing rules related to the synthesis of amorphous structure and the correlations between amorphization and intercalation capability."
More information: Jaehoon Heo et al, Amorphous iron fluorosulfate as a high-capacity cathode utilizing combined intercalation and conversion reactions with unexpectedly high reversibility, Nature Energy (2022). DOI: 10.1038/s41560-022-01148-w
Sung-Kyun Jung et al, Lithium-free transition metal monoxides for positive electrodes in lithium-ion batteries, Nature Energy (2017). DOI: 10.1038/nenergy.2016.208
© 2022 Science X Network