July 28, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Pressure-driven distillation for fast and selective water purification

Membrane technologies can provide efficient purification of unclear water sources to address a growing water shortage. However, when engineers develop state-of-the-art membranes, they are constrained by a lack of selectivity relative to high permeability. Current membranes also only poorly remove low-molecular weight neutral solutes and are vulnerable to degradation due to oxidants inherent to water treatment.
In a new report now published in Science Advances, Duong Nguyen and a research team in environmental and architectural engineering, chemical and biological engineering at the University of Colorado and the University of British Columbia in the U.S., and Canada developed a water desalination method by using applied pressure to drive vapor transport through membranes.
Without sacrificing salt elimination, the team developed membranes with a sub-200-nm thick layer to show water permeabilities that exceeded those of commercial membranes, with sensitivity to chlorine and ozone oxidants. The outcomes led to advanced characterization of the evaporation behavior for high throughput ultra-selective filtration.
Water desalination
Climate change and increasing water demand has led to a scarcity of the resource, necessitating the use of non-traditional sources of water. To safely use the source, researchers must eliminate nearly all dissolved constituents from contaminated water. To accomplish this, several methods such as reverse osmosis have emerged for water reuse and desalination. In this work, Nguyen and colleagues presented a proof-of-concept method to use pressure distillation by trapping air in a hydrophobic membrane.
The applied pressure drove evaporation within the system for gas-phase diffusion through pores. The materials scientists developed nanoporous membranes with sub-200 nm thickness to improve probe transport and desalination performance. This construct allowed the retention of desalination performance, even when the membranes were exposed to chlorine and ozone disinfectants.
Engineering nanoscale air-gaps for pressure-driven distillation
The researchers used air-trapping membranes as a proof-of-concept to test porous anodic aluminum oxide substrates modified with a hydrophobic coat. The team confined the coat to a sub-micron layer on the top surface of the membrane by using masking, metal spluttering and hydrophobic coating with fluorinated alkyl silane. Researchers and civil engineers had not previously used air-trapping membranes in pressurized applications since the liquid-water can enter the pores to compromise water-salt selectivity of the membrane.
The sub-100 nm pore size of the membrane resisted wetting at high hydraulic pressures. Long-term experiments conducted for seven days showed that the salt elimination from membranes could be consistently maintained. The team decreased the membrane pore sizes to function at hydraulic pressures without wetting the pores. These constructs retained pore sizes appropriate for seawater treating.
-
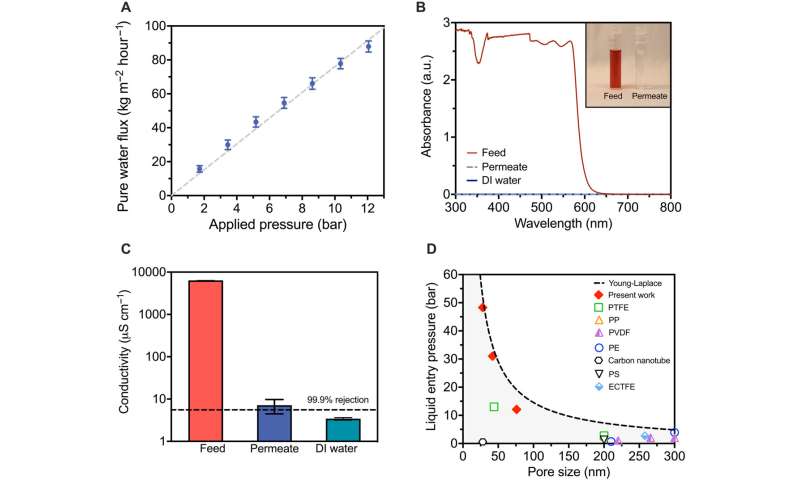
Desalination performance of ultrathin air-trapping membranes. (A) Measured water flux as a function of applied hydraulic pressure for 75.5 ± 14.9 nm pore size membranes with a 189-nm-thick hydrophobic layer. Water flux is normalized to the active pore area of the membrane based on a surface porosity of 14.5%. Feed solution was DI water, temperature was 60°C, and hydraulic pressures varied from 1.72 to 12.1 bar. The dashed line is a linear fit to guide the eye. (B) UV-Vis spectra for 1 mM Allura Red dye feed water, permeate water, and DI water. The inset photo shows the feed and permeate samples. a.u., arbitrary unit. (C) Measured conductivities of the feed, permeate, and DI water for desalination of a 50 mM NaCl solution. The black dashed line depicts 99.9% salt rejection. The operating pressure and temperature were 10.3 bar and 60°C, respectively. (D) LEP as a function of pore size for the membranes fabricated in this work and membranes reported in the literature: commercial membranes made of polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), polypropylene (PP), and polyethylene (PE) and fabricated membranes made of carbon nanotubes, nanofibrous polystyrene (PS), and poly(ethylene chlorotrifluoroethylene) (ECTFE). The dashed curve presents theoretical values obtained from the Young-Laplace equation with an intrinsic contact angle of 120°. In all plots, error bars denote ±1 SD around the mean from three separate membranes. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638 -
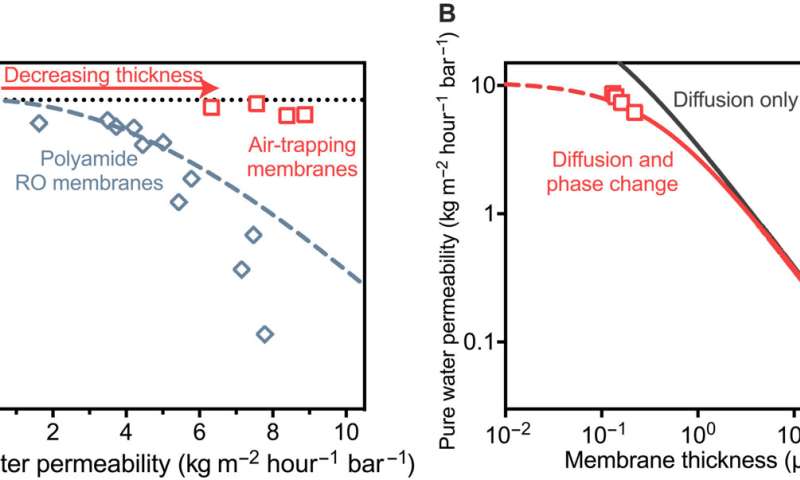
Achievable water permeability in air-trapping membranes. (A) Normalized pure water permeability and sodium chloride rejection of air-trapping membranes (red squares). The permeability of air-trapping membranes was increased by decreasing the thickness of the air layer from 100 μm to 119 nm. Permeability values of air-trapping membranes are normalized to the active pore area based on a surface porosity of 14.5%. Membranes were tested with 50 mM NaCl at a temperature of 60°C and an applied pressure of 10.3 bar. For comparison, the permeability-selectivity trade-off curve for polyamide membranes is shown from experimental measurements (blue diamonds) and theoretical estimations (dotted blue line). (B) Water permeability of air-trapping membranes as a function of membrane thickness (red squares). Simulated curves based on only diffusion resistances or diffusion and phase-change resistances are shown. Phase-change resistances were modeled using a condensation coefficient of 0.33. Dashed lines indicate thicknesses below the minimum thickness that causes pore wetting. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638
Achieving water permeability and salt elimination
The existing state-of-the-art membranes present a tradeoff between water permeability and water-salt selectivity, whereby a gain in water permeability led to a loss in salt rejection. In this work, the team noted a how a gas-liquid phase change can increase water permeability without sacrificing the water-salt selectivity by decreasing the thickness of the air layer.
Due to this feature, water permeabilities of membranes were notably higher than in previous work. The scientists exceeded the expectations of this study when they showed experimental evidence for the possibilities of air-trapping membranes to reach water permeabilities suited for efficient water desalination.
Solute elimination and chemical resilience
The research team used a vapor layer as a separation barrier with different selective properties as opposed to the incorporation of a thin polymer film. Using this membrane, they tested the removal of three contaminants including boron, urea and NDMA. The membranes accomplished 99.1% and 98.1% elimination of boron and urea respectively to highlight near-complete removal of both solutes. The outcomes contrasted with commercial membranes that showed substantially decreased filtration levels.
The improved results were due to the air layer that formed a near-impermeable filter membrane for non-volatile solutes. The air layer allowed Nguyen and colleagues to use oxidation-resistant hydrophobic membrane materials. The scientists interestingly confirmed the unchanged structure and chemistry of the engineered membranes after exposing them to scanning electron microscopy and contact angle analysis.
-
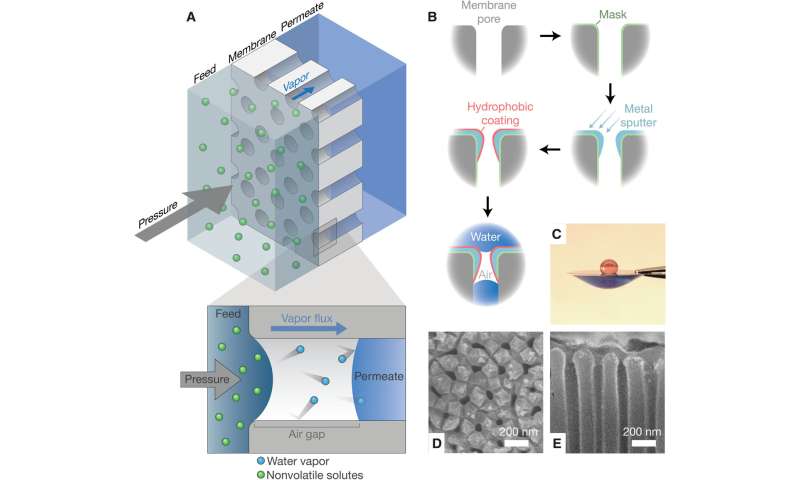
Design of ultrathin air-trapping membranes for pressure-driven vapor transport. (A) Schematic diagram of pressure-driven water vapor transport through a nanoporous membrane with an ultrathin air gap. (B) Schematic of the fabrication process: A porous alumina membrane is modified with a hydrophilic masking layer, sputtered with controlled metal deposition into pores, selectively coated with a hydrophobic layer on the metal surface, and treated to remove the residual masking layer. (C) Water contact angle on the top and bottom surfaces of the membrane. SEM of the (D) top surface and (E) cross section of the upper surface of the membrane. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638 -
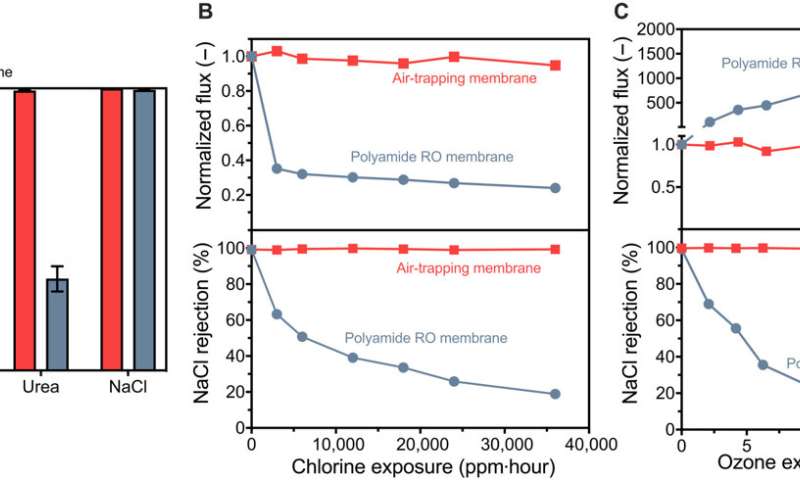
Selectivity and oxidation resistance of fabricated membranes. (A) Elimination of boron, NDMA, urea, and sodium chloride by the fabricated air-trapping membranes with a pore size of 75.5 ± 14.9 nm and commercial polyamide RO membranes (Dow SW30). Error bars indicate SD for duplicate experiments using two membranes fabricated in the same procedure. (B) Effect of chlorine exposure on the water flux and salt rejection of the fabricated air-trapping membranes and polyamide RO membranes. Chlorine concentration is 1000 ppm at pH 4, and exposure time varies from 1 to 36 hours. (C) Effect of ozone exposure on membrane water flux and salt rejection. Ozone concentration is 25 ppm at pH 7, and exposure time varies from 0.083 to 1 hour. The applied hydraulic pressure was fixed at 10.3 bar for all experiments. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg6638
Outlook
In this way, Duong Nguyen and colleagues used a pressure-driven distillation process for water purification with applied pressure used to drive vapor through an air-trapping membrane. By using proof-of-concept experiments, the scientists achieved higher filtration levels of nonvolatile solutes, including sodium chloride, boron and urea. The material's performance remained unimpaired upon exposure to sustained levels of high concentrations of chlorine and ozone.
This work outlines the fundamental principles of pressure-driven distillation methods, although gaps still exist in the knowledge of the underlying evaporation process. These membranes provide a platform to broadly study the evaporation phenomena at the air-membrane interface.
More information: Duong T. Nguyen et al, Pressure-driven distillation using air-trapping membranes for fast and selective water purification, Science Advances (2023). DOI: 10.1126/sciadv.adg6638
Menachem Elimelech et al, The Future of Seawater Desalination: Energy, Technology, and the Environment, Science (2011). DOI: 10.1126/science.1200488
© 2023 Science X Network