This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New reactor could save millions when making ingredients for plastics and rubber from natural gas

A new way to make an important ingredient for plastics, adhesives, carpet fibers, household cleaners and more from natural gas could reduce manufacturing costs in a post-petroleum economy by millions of dollars, thanks to a new chemical reactor designed by University of Michigan engineers.
The reactor creates propylene, a workhorse chemical that is also used to make a long list of industrial chemicals, including ingredients for nitrile rubber found in automotive hoses and seals as well as blue protective gloves. Most propylene used today comes from oil refineries, which collect it as a byproduct of refining crude oil into gasoline.
As oil and gasoline fall out of vogue in favor of natural gas, solar, and wind energy, production of propylene and other oil-derived products could fall below the current demand without new ways to make them.
Natural gas extracted from shale holds one potential alternative to propylene sourced from crude oil. It's rich in propane, which resembles propylene closely enough to be a promising precursor material, but current methods to make propylene from natural gas are still too inefficient to bridge the gap in supply and demand.
"It's very hard to economically convert propane into propylene," said Suljo Linic, the Martin Lewis Perl Collegiate Professor of Chemical Engineering and the corresponding author of the study published in Science.
"You need to heat that reaction to drive it, and standard methods require very high temperatures to produce enough propylene. At those temperatures, you don't just get propylene but solid carbon deposits and other undesirable products that impair the catalyst. To regenerate the reactor, we need to burn off the solid carbon deposits often, which makes the process inefficient."
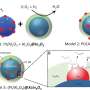
The researchers' new reactor system efficiently makes propylene from shale gas by separating propane into propylene and hydrogen gas. It also gives hydrogen a way out, changing the balance between the concentration of propane and reaction products in a way that allows more propylene to be made. Once separated, the hydrogen can also be safely burned away from the propane, heating the reactor enough to speed up the reactions without making any undesirable compounds.
This separation is achieved through the reactor's nested, hollow-fiber membrane tubing. The innermost tube is made up of materials that splits the propane into propylene and hydrogen gas. While the tubing keeps most of the propylene inside the innermost chamber, the hydrogen gas can escape into an outer chamber through pores in a membrane layer of the material. Inside that chamber, the hydrogen gas is controllably burned by mixing in precise amounts of oxygen.
Because the hydrogen can be burned inside the reactor and can operate under higher propane pressures, the technology could allow plants to produce propylene from natural gas without installing extra heaters. A plant that produces 500,000 metric tons of propylene annually could save as much as $23.5 million over other methods starting with shale gas, according to the researchers' estimates. Those savings come on top of the operational savings from burning hydrogen produced in reaction, rather than other fuels.
More information: Rawan Almallahi et al, Overcoming limitations in propane dehydrogenation by co-designing catalysts/membrane systems, Science (2024). DOI: 10.1126/science.adh3712. www.science.org/doi/10.1126/science.adh3712