July 12, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new approach to boost the efficiency of non-fused ring electron acceptor solar cells
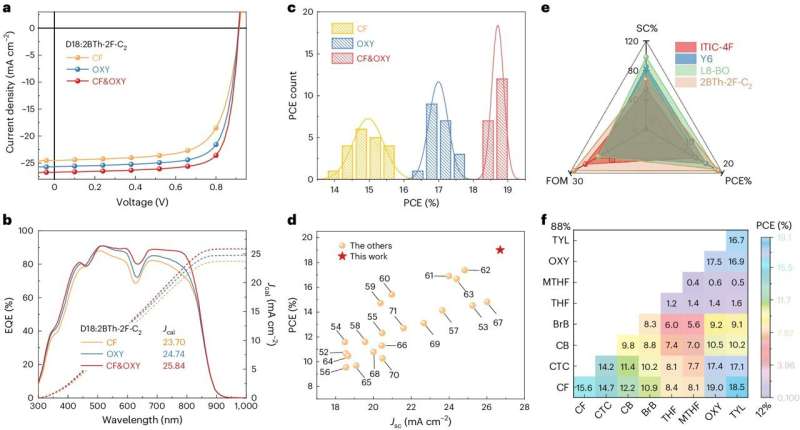
The power-conversion efficiencies (PCEs) of organic solar cells based on compounds known as polymer donors and fused ring electron acceptors (FREAs) have recently exceeded 19%. In contrast, organic solar cells based on non-fused ring electron acceptors (NFREAs), more affordable compounds characterized by non-fused (i.e., separate) aromatic rings, have so far exhibited disappointing efficiencies of around 16%.
As synthesizing NFREAs is significantly less expensive than synthesizing FREAs, developing more efficient solar cells based on these materials could have important implications. Specifically, it could facilitate the widespread adoption of organic solar cells, thus potentially contributing to the reduction of emissions and the mitigation of environmental issues.
Researchers at Shanghai Jiao Tong University, Qingdao University and other institutes in China recently proposed a new approach to fabricate more efficient organic solar cells based on NFRAs. This approach, outlined in a paper published in Nature Energy, relies on the use of a solvent based on chloroform (CF) and o-xylene (OXY), as well as a solid-state additive that further enhances crystallization in NFRAs, thus enabling higher PCEs in solar cells based on these compounds.
"Non-fused ring electron acceptors (NFREAs) potentially have lower synthetic costs than their fused counterparts," Rui Zeng, Ming Zhang and their colleagues wrote in their paper. "However, the low backbone planarity and the presence of bulky substituents adversely affect the crystallinity of NFREAs, impeding charge transport and the formation of bicontinuous morphology in organic solar cells. We show that a binary solvent system can individually control the crystallization and phase separation of the donor polymer (for example, D18) and the NFREA (for example, 2BTh-2F-C2)."
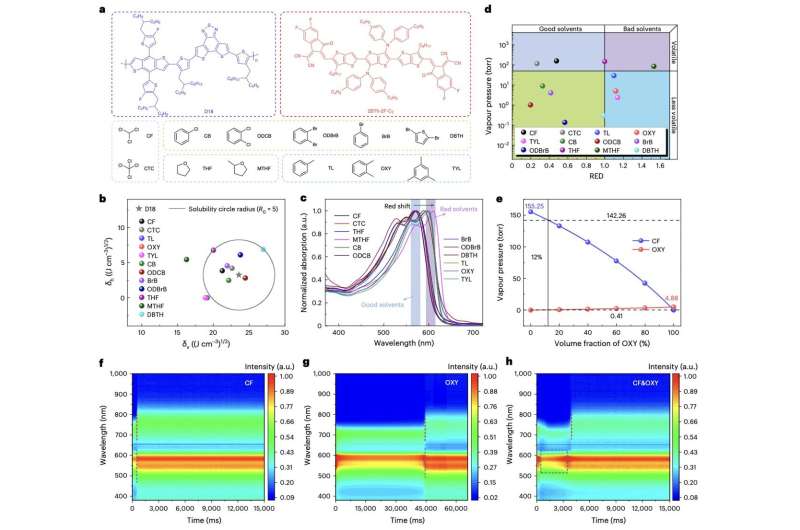
As part of their study, Zeng, Zhang and their collaborators first designed and synthesized a compound mixture containing CF and OXY. They then observed how a donor polymer and NFREA responded to this solvent mixture, focusing specifically on the formation of films on these compounds.
"We select solvents such as CF and OXY that evaporate at different temperatures and rates and have different solubility for the donor polymer D18," the researchers wrote. "Upon evaporation of chloroform, D18 starts to assemble into fibrils. Then, the evaporation of o-xylene induces the rapid formation of a fibril network that phase segregates 2BTh-2F-C2 into pure domains and leads to a bicontinuous morphology."
The researchers also introduced a solid-state additive, namely 1,4-diiodobenzene (DIB), to their sample. This additive was placed in the formed photoactive thin film, while it had almost dried, to further enhance the crystallization of the NFREA.
The researchers used their approach to develop new solar cells based on NFREAs, which they then assessed in a series of initial tests. Remarkably, they found that the morphology enabled by their solvent and additive enabled PCEs of 19.02% for small-area (0.052cm2) cells and 17.28% for 1 cm2 devices.
This recent study opens new possibilities for the fabrication of organic solar cells based on NFREAs, which could be significantly less expensive than their FREAs-based counterparts. The promising findings gathered by this research team could soon inspire further efforts in this direction, potentially contributing to the future commercialization of organic solar cells.
More information: Rui Zeng et al, Achieving 19% efficiency in non-fused ring electron acceptor solar cells via solubility control of donor and acceptor crystallization, Nature Energy (2024). DOI: 10.1038/s41560-024-01564-0
© 2024 Science X Network