This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Recovering valuable materials with better membranes

Critical minerals like lithium and cobalt are vital to batteries, electric vehicles, and renewable energy systems. To meet the long-term demand for these materials, finding sources of these materials beyond mining has become a priority for many researchers.
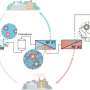
To that end, membrane technology has shown great promise in extracting these materials from alternate sources. Featuring nanosized pores, these membranes can be tuned to filter specific materials from water and other sources.
In a paper in Nature Water, a team of researchers from Yale University and MIT has drawn inspiration from living organisms to outline a way to hone this technology.
"There's a recent movement in membrane science to design at a more precise level," said Camille Violet, lead author of the paper. "We need to think more like biochemists and design at the molecular level so that we can separate chemically similar species."
For instance, cobalt and nickel are right next to each other on the periodic table. That makes it difficult for conventional membranes, which separate species based on differences in size or charge, to separate these near-identical species.
"Some of these really important metals that we need to separate only differ by one atomic number," said Violet, a Ph.D. candidate in the lab of Menachem Elimelech, the Sterling Professor of Chemical and Environmental Engineering.
"As the technologies and products we use have become more complex, the separation challenges involved in recovering metals from those waste streams have become more difficult. We need to be designing our separation technologies with greater precision."
In their perspective article, the researchers detailed a plan on how to reach this level of detail.
"It lays out a roadmap from identifying selective chemical groups that we can use to separate similar species to incorporating them into membrane design."
Doing so could have major practical benefits. Some of the most important materials right now are the critical metals used in batteries, such as cobalt, nickel, manganese, lithium. Extracting these metals, though, has a huge environmental cost and often involves unethical human labor practices.
As an alternative source, untapped resources abound in waste streams. Electronic waste contains rare earth elements and precious metals, spent lithium-ion batteries contain cobalt and lithium, and municipal and industrial wastewater hosts many valuable products ranging from pharmaceuticals to fertilizers.
"So we really need to develop alternative routes to recover those materials," Violet said.
"But we need to figure out how to separate them into their pure components from complex waste streams. Membranes would be a huge leap forward in resource recovery for cathode materials for batteries. They could also be hugely beneficial in separating nitrate from agricultural runoff and advancing water treatment. As society modernizes, our waste streams get more complex, and we need to start processing them at greater resolution."
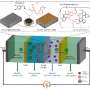
To do so, the researchers draw inspiration from life forms. For instance, nearly all organisms have potassium ion channels, which can exclude sodium to transport potassium.
"If we look to the biological ion channel as a model, then we can start to mimic that design in membrane science. To do that, we'll need to figure out how to select a binding site that is selective for our target ion, and how we should pattern those binding sites within the membrane material so that we can achieve fast transport as well."
One goal of the paper, she said, is to get membrane scientists to think more like biochemists.
"Biochemistry is so much more advanced than membrane science when it comes to knowledge of selective chemical interactions," she said.
"Metalloproteins already selectively bind these metals, so we can look to protein databases for examples. We can draw inspiration from pharmaceutical development, and we can use drug discovery models to try to design channels in synthetic membranes."
More information: Camille Violet et al, Designing membranes with specific binding sites for selective ion separations, Nature Water (2024). DOI: 10.1038/s44221-024-00279-6