Using water to reduce carbon dioxide emissions
Water could play a crucial role in reducing global carbon dioxide (CO2) emissions, according to Johns Hopkins engineers.
Sep 5, 2023
1
58
Engineering

Water could play a crucial role in reducing global carbon dioxide (CO2) emissions, according to Johns Hopkins engineers.
Sep 5, 2023
1
58
Energy & Green Tech

Researchers at the University of Colorado have developed a new and efficient way to produce green hydrogen or green syngas, a precursor to liquid fuels. The findings could open the door for more sustainable energy use in ...
Aug 17, 2023
0
123
Energy & Green Tech
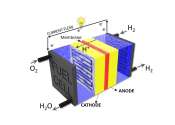
Scientists at the University of New South Wales Sydney are working on ways to improve the efficiency and cost of hydrogen fuel cells, to increase access to clean fuel.
Aug 11, 2023
0
89
Energy & Green Tech

A team from the University of Toronto's Faculty of Applied Science & Engineering has invented a device that leverages electrochemistry to increase the efficiency of direct air carbon capture. Their alternative strategy aims ...
Aug 10, 2023
0
243
Energy & Green Tech

Efficient production of hydrogen, fuels, and even drinking water on roofs or in solar parks—this is what researchers of Karlsruhe Institute of Technology (KIT) and their Canadian partners want to achieve with low-cost photoreactor ...
Jul 4, 2023
1
115
Energy & Green Tech

A research team led by Professor Yong-Tae Kim (Department of Material Science and Engineering and Graduate Institute of Ferrous and Energy Materials Technology) and Ph.D. candidate Sang-Hoon You (Department of Material Science ...
May 12, 2023
0
59
Energy & Green Tech

As the world faces an increasing demand for clean and sustainable energy sources, scientists are turning to the power of photosynthesis for inspiration. With the goal of developing new, environmentally friendly techniques ...
May 10, 2023
0
83
Electronics & Semiconductors

In the effort to reduce our reliance on fossil fuels, one strategy involves harvesting the waste heat that is already being produced by our energy systems. Thermoelectric generators can convert waste heat to clean electricity, ...
May 1, 2023
0
49
Electronics & Semiconductors

Emerging AI applications, like chatbots that generate natural human language, demand denser, more powerful computer chips. But semiconductor chips are traditionally made with bulk materials, which are boxy 3D structures, ...
Apr 27, 2023
0
122
Energy & Green Tech

A recycling method developed by Karlsruhe Institute of Technology (KIT) recovers up to 70% of lithium from battery waste without corrosive chemicals, high temperatures, and prior sorting of materials being required. The method ...
Mar 30, 2023
0
154
A chemical reaction is a process that always results in the interconversion of chemical substances. The substance or substances initially involved in a chemical reaction are called reactants. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Classically, chemical reactions encompass changes that strictly involve the motion of electrons in the forming and breaking of chemical bonds, although the general concept of a chemical reaction, in particular the notion of a chemical equation, is applicable to transformations of elementary particles, as well as nuclear reactions. Different chemical reactions are used in combination in chemical synthesis in order to get a desired product. In biochemistry, series of chemical reactions catalyzed by enzymes form metabolic pathways, by which syntheses and decompositions ordinarily impossible in conditions within a cell are performed.
This text uses material from Wikipedia, licensed under CC BY-SA