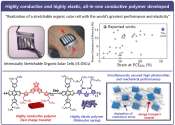
A polymer (from Greek πολύ-ς /po΄li-s/ much, many and μέρος /΄meros/ part) is a large molecule (macromolecule) composed of repeating structural units typically connected by covalent chemical bonds. While polymer in popular usage suggests plastic, the term actually refers to a large class of natural and synthetic materials with a variety of properties.
Due to the extraordinary range of properties accessible in polymeric materials , they have come to play an essential and ubiquitous role in everyday life - from plastics and elastomers on the one hand to natural biopolymers such as DNA and proteins that are essential for life on the other. A simple example is polyethylene, whose repeating unit is based on ethylene (IUPAC name ethene) monomer. Most commonly, as in this example, the continuously linked backbone of a polymer consists mainly of carbon atoms. However, other structures do exist; for example, elements such as silicon form familiar materials such as silicones, examples being silly putty and waterproof plumbing sealant. The backbone of DNA is in fact based on a phosphodiester bond, and repeating units of polysaccharides (e.g. cellulose) are joined together by glycosidic bonds via oxygen atoms.
Natural polymeric materials such as shellac, amber, and natural rubber have been in use for centuries. Biopolymers such as proteins and nucleic acids play crucial roles in biological processes. A variety of other natural polymers exist, such as cellulose, which is the main constituent of wood and paper.
The list of synthetic polymers includes synthetic rubber, Bakelite, neoprene, nylon, PVC, polystyrene, polyacrylonitrile, PVB, silicone, and many more.
Polymers are studied in the fields of polymer chemistry, polymer physics, and polymer science.