Sun, sustainability, and silicon: A double dose of solar fuel research
The race is on to develop a new generation of liquid fuels that are activated by sunlight, and Yale researchers are helping to lead the way.
May 17, 2024
0
25
Energy & Green Tech

The race is on to develop a new generation of liquid fuels that are activated by sunlight, and Yale researchers are helping to lead the way.
May 17, 2024
0
25
Machine learning & AI

Top US lawmakers on Wednesday said efforts to pass laws governing AI were entering a higher gear and hoped to pump $32 billion into the sector to help assure US dominance.
May 15, 2024
0
37
Business

In the battle to build software-driven connected vehicles, General Motors Co. CEO Mary Barra knows the "industry is so fierce, you have to have the very best talent."
May 15, 2024
0
1
Engineering

Roof tiles are becoming a thing of the past: Today, more and more Swiss roofs boast large black and blue rectangles that convert sunlight into electricity. The blueish color comes from silicon crystals, as the majority of ...
May 2, 2024
0
1
Hardware

A smartphone shutting down on a sweltering day is an all-too-common annoyance that may accompany a trip to the beach on a sunny afternoon. Electronic memory within these devices isn't built to handle extreme heat.
Apr 30, 2024
0
72
Engineering
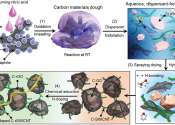
Researchers have developed a new manufacturing technique for "silicon/nitrogen-doped carbon composite anode materials." These materials aim to enhance the capacity and stability of lithium-ion battery anodes.
Apr 24, 2024
0
1
Hardware

The mass production of conventional silicon chips relies on a successful business model with large "semiconductor fabrication plants" or "foundries." New research by KU Leuven and imec shows that this "foundry" model can ...
Apr 24, 2024
0
30
Business

Karim Beguir launched the artificial intelligence start-up InstaDeep in Tunisia in 2014 with just two computers and $2,000.
Apr 21, 2024
0
3
Business

Taiwanese semiconductor giant TSMC announced Thursday a nearly 9 percent increase in net profits in the first quarter of 2024, buoyed by global demand for its microchips used to power everything from mobile phones to AI technology.
Apr 18, 2024
0
12
Machine learning & AI

South Korea will invest almost $7 billion in artificial intelligence by 2027 in an effort to become a global leader in cutting-edge semiconductors, President Yoon Suk Yeol said Tuesday.
Apr 9, 2024
0
10
Silicon (pronounced /ˈsɪlɨkən/ or /ˈsɪlɨkɒn/, Latin: silicium) is the most common metalloid. It is a chemical element, which has the symbol Si and atomic number 14. The atomic mass is 28.0855. A tetravalent metalloid, silicon is less reactive than its chemical analog carbon. As the eighth most common element in the universe by mass, silicon very rarely occurs as the pure free element in nature, but is more widely distributed in dusts, planetoids and planets as various forms of silicon dioxide (silica) or silicates. On Earth, silicon is the second most abundant element (after oxygen) in the crust, making up 25.7% of the crust by mass.
Silicon has many industrial uses. It is the principal component of most semiconductor devices, most importantly integrated circuits or microchips. Silicon is widely used in semiconductors because it remains a semiconductor at higher temperatures than the semiconductor germanium and because its native oxide is easily grown in a furnace and forms a better semiconductor/dielectric interface than any other material.
In the form of silica and silicates, silicon forms useful glasses, cements, and ceramics. It is also a constituent of silicones, a class-name for various synthetic plastic substances made of silicon, oxygen, carbon and hydrogen, often confused with silicon itself.
Silicon is an essential element in biology, although only tiny traces of it appear to be required by animals. It is much more important to the metabolism of plants, particularly many grasses, and silicic acid (a type of silica) forms the basis of the striking array of protective shells of the microscopic diatoms.
This text uses material from Wikipedia, licensed under CC BY-SA