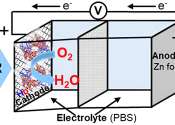
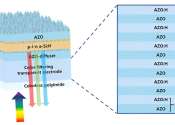
Zinc (pronounced /ˈzɪŋk/, from German: Zink and also known as spelter) is a metallic chemical element with the symbol Zn and atomic number 30. It is a first-row transition metal in group 12 of the periodic table. Zinc is chemically similar to magnesium because its ion is of similar size and its only common oxidation state is +2. Zinc is the 24th most abundant element in the Earth's crust and has five stable isotopes. The most exploited zinc ore is sphalerite, or zinc sulfide; the largest exploitable deposits are found in Australia, Canada and the United States. Zinc production includes froth flotation of the ore, roasting and final extraction using electricity.
Brass, which is an alloy of copper and zinc, has been used since at least the 10th century BC. Impure zinc metal was not produced in large scale until the 13th century in India, while the metal was unknown to Europe until the end of the 16th century. Alchemists burned zinc in air to form what they called "philosopher's wool" or "white snow." The element was probably named by the alchemist Paracelsus after the German word Zinke. German chemist Andreas Sigismund Marggraf is normally given credit for discovering pure metallic zinc in a 1746 experiment. Work by Luigi Galvani and Alessandro Volta uncovered the electrochemical properties of zinc by 1800. Corrosion-resistant zinc plating of steel is the major application for zinc. Other applications are in batteries and alloys, such as brass. A variety of zinc compounds are commonly used, such as zinc chloride (in deodorants), zinc pyrithione (anti-dandruff shampoos), zinc sulfide (in luminescent paints), and zinc methyl or zinc diethyl in the organic laboratory.
Zinc is an essential mineral of "exceptional biologic and public health importance". Zinc deficiency affects about 2 billion people in the developing world and is associated with many diseases. In children it causes growth retardation, delayed sexual maturation, infection susceptibility, and diarrhea, contributing to the death of about 800,000 children worldwide per year. Enzymes with a zinc atom in the reactive center are widespread in biochemistry, such as alcohol dehydrogenase in humans. Consumption of excess zinc can cause ataxia, lethargy and copper deficiency.