October 29, 2019 feature
Batteries with fluorinated electrolytes that work at very high and low temperatures
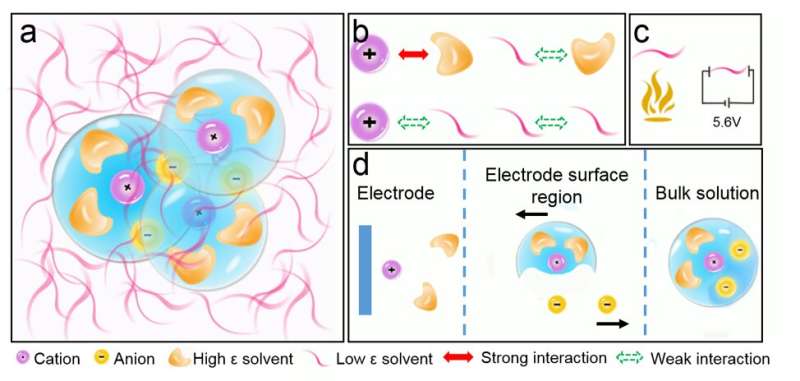
Electrolytes are chemical components that enable the flow of ions between the cathode and anode inside batteries, ultimately providing electrical power to technological devices. Most conventional and readily available non-aqueous Li-ion batteries are fabricated using carbonate-based electrolytes.
Despite their widespread use, the highly flammable carbonate electrolytes greatly limit the temperatures at which a battery can operate correctly due to their high affinity between their chemical solvents and the ions inside batteries. This results in most carbonate electrolytes-based batteries only functioning safely between -20°C and +50°C, or at voltages between 0.0 and 4.3V.
With this in mind, a team of researchers led by professor Chunsheng Wang at the University of Maryland in the US and other scientists at Zhejiang University in China have recently fabricated a new type of battery that can work at a broader range of temperatures, using fluorinated electrolytes with non-polar solvents. These fluorinated electrolytes are non-flammable, enabling a high electrochemical stability within a broader range temperatures and voltages than carbonate electrolytes.
"In current electrolytes, the electrochemical stability window and operation temperature window cannot reach the maximum at the same time due to intrinsic limitation of solvation structure of the electrolytes," Xiao Ji, one of the researchers who carried out the study, told TechXplore. "By reducing the Li-ion and bonding with solvent through adding an antisolvent, we decoupled the electrochemical and physical property of the electrolytes and developed all temperature (-95oC to +60oC) and all voltage (from 0.0V to 5.6V) Li-ion battery electrolytes." said of Dr. Xiulin Fan, the first author of the paper.
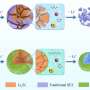
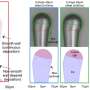
Essentially, Ji and his colleagues were able to tame the affinity between chemical solvents and Li ions in batteries by dissolving fluorinated electrolytes into highly fluorinated non-polar solvents (i.e. solvents containing bonds between atoms with similar electronegativities). The electrolytes they used enable a high electrochemical stability in a wide voltage window of 0.0 to 5.6 V, as well as high ionic conductivities within a wide temperature range between -125 and +70 °C.
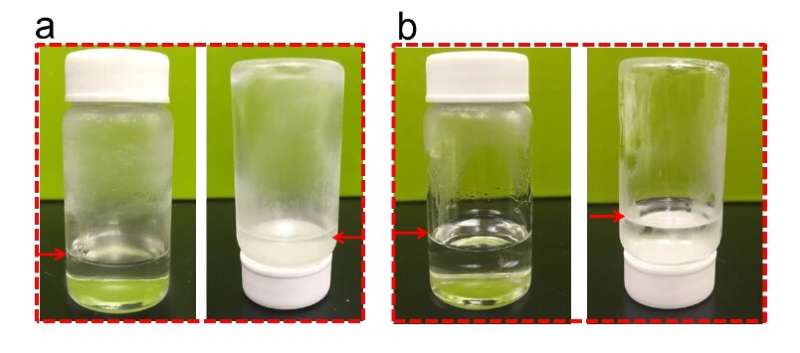
With the fluorinated electrolytes, the researchers found that LiNi0.8Co0.15Al0.05O2 cathodes achieved high Coulombic efficiencies of 99.9 percent, between −95 and + 70 °C, while aggressive Li anodes and the high-voltage (5.4 V) LiCoMnO4 achieved Coulombic efficiencies of 99.4 percent and 99 percent, respectively. Moreover, even at −85 °C, the battery could still deliver ~50 percent of its room-temperature capacity.
"The Li ion transport barriers is dramatically reduced in our electrolyte, the liquid range of the electrolyte is greatly widened," Chunsheng Wang said. "In addition, the developed electrolyte can withstand a much higher voltage compared to the conventional commercialized carbonate electrolytes. Therefore, the batteries based on our electrolyte can work within a much wider temperature range."
The fluorinated electrolytes used by Wang and his colleagues have so far proved to be more effective than carbonate electrolytes, attaining a high electrochemical stability within a wider voltage window and high ionic conductivities at a wider range of temperatures. As they are entirely non-flammable, they are also far safer than carbonate electrolytes. In the future, the electrolytes proposed by this team of researchers could be used to build highly-performing batteries that can also function in extreme climates, for example in the Arctic or in the African savannah.
"We will now try to optimize the composition of the batteries we developed to reduce their cost and also collaborate with battery industries to commercialize the all-temperature batteries," Fan added.
More information: Xiulin Fan et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents, Nature Energy (2019). DOI: 10.1038/s41560-019-0474-3
© 2019 Science X Network