July 28, 2020 feature
Lithium metal batteries that perform well at low temperatures
Lithium (Li) batteries, or lithium metal batteries, use metallic lithium as an anode. Over the past few decades, rechargeable Li batteries have been used to power a wide variety of electronic devices, including toys, portable consumer devices and electric vehicles.
While these batteries typically achieve reliable performances at room temperature, their energy efficiency, power and cycle life tend to decrease significantly at temperatures below -10 °C. The inability to function well at low temperatures is a crucial drawback, as it greatly limits their use in regions with particularly cold climates. The main reason for this limitation is that at temperatures below -10 °C the solid-electrolyte interphase (SEI) becomes unstable and leads to what is known as the dendritic Li plating of the anode in the batteries.
A team of researchers at Pennsylvania State University and Argonne National Laboratory recently introduced a new design for Li metal batteries that could overcome this well-documented drawback. The resulting batteries, presented in a paper published in Nature Energy, were found to perform remarkably well at low temperatures compared to previously developed Li batteries.
"We report on high-performance Li metal batteries under low-temperature and high-rate-charging conditions," the researchers wrote in their paper. "The high performance is achieved by using a self-assembled monolayer of electrochemically active molecules on current collectors that regulates the nanostructure and composition of the SEI and deposition morphology of Li metal anodes."
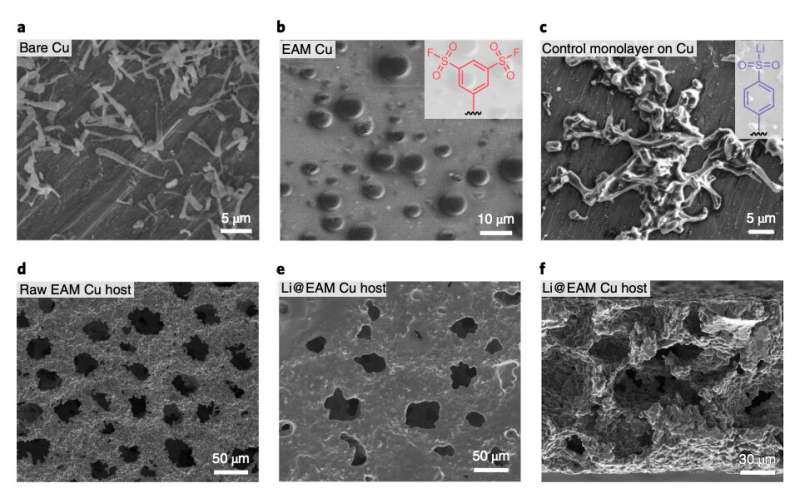
Initially, the researchers closely examined Li metal batteries at low temperatures in order to better understand the factors impairing their performance. They observed that at -15 °C the battery's SEI, derived from a conventional electrolyte, is highly crystalline and inhomogeneous. This greatly limits the formation of passive SEI components such as lithium fluoride nanosalts, leading to unfavorable characteristics such as poor surface passivation, Li corrosion and the growth of dendrites on the anode.
Adding layers to protect the anode, using alternative electrolytes or introducing Li hosts can prevent these effects at room temperature, but at low temperatures, controlling SEI nanostructures is far more challenging, leading to unstable battery operation. The researchers thus devised an approach for creating an SEI that is passive at the nanoscale level, which could in turn enable the stable operation of Li metal anodes at low temperatures.
The approach presented in their paper entails the regulation of the SEI nanostructure and Li nucleation in a Li battery using a monolayer of 1,3-benzenedisulfonyl fluoride assembled on the surface of copper current collectors. This newly introduced, electrochemically active monolayer (EAM) changes the chemical environment in the interface, prompting the formation of lithium fluoride (LiF) nuclei on the Li surface.
By changing the battery's interfacial chemical environment, the new design strategy introduced by the researchers alters the pathway and dynamics of electrolyte decomposition, which in turn leads to the production of a different SEI with enhanced passivation qualities. More specifically, the monolayer forms a lithiophilic anion on the Cu current collectors (i.e., benzenesulfinate), which can guide Li nucleation and growth when there is a low Li ion concentration in the interface.
At low temperatures, this design strategy leads to the formation of a multi-layered SEI composed of an LiF-rich inner phase and an amorphous outer layer. This passive, multi-layered SEI differs greatly from the non-passive ones observed in conventional Li metal batteries, which are characterized by a highly crystalline and Li2CO3-dominant structure at low temperatures.
Under testing, the batteries they created using the new design strategy achieved remarkable results at low temperatures. More specifically, their approach led to the successful suppression of galvanic Li corrosion and self-discharge, enabling the stable deposition of Li at all temperatures ranging between -60 and 45°C.
The researchers found that a Li | LiCoO2 cell with a capacity of 2.0 mAh cm−2 fabricated using their method could achieve a 200-cycle life at -15 °C, with a recharge time of 45 min. In the future, the design they introduced could pave the way for the creation of new energy-efficient lithium batteries that can operate reliably in countries or geographical areas where temperatures drop below -10 or -15 °C.
More information: Yue Gao et al. Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface, Nature Energy (2020). DOI: 10.1038/s41560-020-0640-7
© 2020 Science X Network