November 16, 2020 feature
Designing layered oxide materials for sodium-ion batteries
Lithium cobalt oxide is a layered metal oxide that has attracted great attention to develop rechargeable batteries. Sodium-ion batteries can store grid-scale energy due to the natural abundance of sodium. The composition can determine the structural chemistry for electrochemical performance, however, due to complex compositions, the results are very challenging to predict. In a new report now on Science, Chenglong Zhao and a team of international scientists at the Chinese Academy of Science, China, Harvard University, U.S., Delft University of Technology, the Netherlands, and the University of Toronto, Canada, introduced a "cationic potential" to capture key interactions of layered materials and predict the resulting stacking structures. The team showed how the stacking architecture determined the functional properties to offer solutions towards developing alkali metal layered oxides for electrical energy storage.
Layering the charge
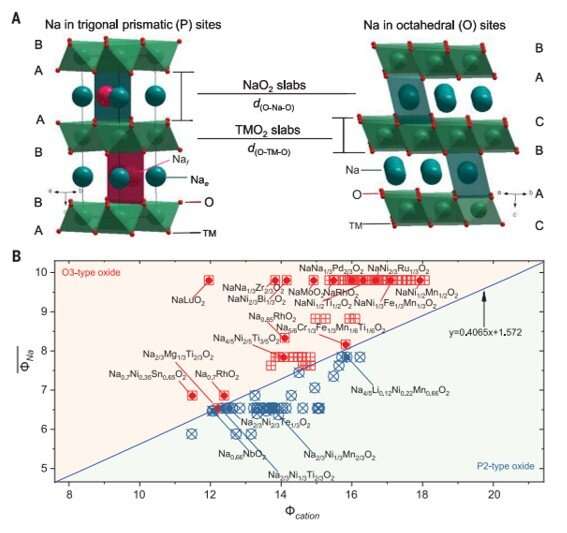
Researchers must develop sustainable electrical energy storage systems to meet the demands to integrate intermittent, renewable energy. When compared with lithium (Li)-ion batteries, the abundance and low cost of sodium (Na) make Na-ion batteries promising substituents for smart grids and large-scale energy storage. Since 1980, Li-ion layered batteries have represented the dominant family of electrode materials; incidentally developed as a new cathode material for which American materials scientist J.B.Goodenough eventually received the joint Nobel Prize in Chemistry with M. Stanley Whittingham and Akira Yoshino, in 2019. One or multiple transition metal (TM) elements can facilitate the redox reaction associated with Li-ion (de-)intercalation. For example, materials scientists can build layered structures from edge-sharing transition metal oxide (TMO6) octahedra to form repeating layers, amongst which lithium ions can be positioned in the octahedral oxygen environment to observe O-type stacking. This architecture offers high compositional diversity and tunable electrochemical performance with improved charge.
![Designing an O3-type oxide. (A) Analysis of the cationic potential of Na-Li-Mn(Ti)-O oxides. (B) XRD patterns of the targeted NaLi1/3Mn2/3O2 and the standard references. (C) Rietveld refinement of XRD pattern of NaLi1/3Ti1/6Mn1/2O2. (D) Schematic illustration of the corresponding structure with the Li/Mn(Ti) ordering in the [Li1/3Ti1/6Mn1/2]O2 slabs. Credit: Science, doi: 10.1126/science.aay9972 Designing layered oxide materials for sodium-ion batteries](https://scx1.b-cdn.net/csz/news/800a/2020/1-designinglay.jpg)
Layered oxides offer a natural starting point in the search for electrodes for sodium (Na)-ion batteries. However, aside from O-type stacking for Na-ion oxides, P-type stacking can also occur, where P-type refers to prismatic Na-ion coordination with distinctly different electrode performance. Researchers have investigated a variety of P2-type (hexagonal) and O3-type (rhombohedral) sodium-ion layered oxides to search for electrodes with good chemical and dynamic stability, as well as high Na storage performance. Nevertheless, effective guidelines are not yet in place to design and prepare such optimal electrode materials suited for sodium-ion batteries.
![Designing a P2-type oxide. (A) Analysis of cationic potential of Na-Li-Mn-O oxides. (B) XRD patterns of NaLi1/3Ti1/6Mn1/2O2 and Na5/6Li5/18Mn13/18O2 oxides. (C) Rietveld refinement of XRD pattern of Na5/6Li5/18Mn13/18O2. (D) Schematic illustration of the corresponding structure with the Li/Mn ordering in the [Li5/18Mn13/18]O2 slabs. Credit: Science, doi: 10.1126/science.aay9972 Designing layered oxide materials for sodium-ion batteries](https://scx1.b-cdn.net/csz/news/800a/2020/2-designinglay.jpg)
Using the cationic potential to design sodium ion batteries
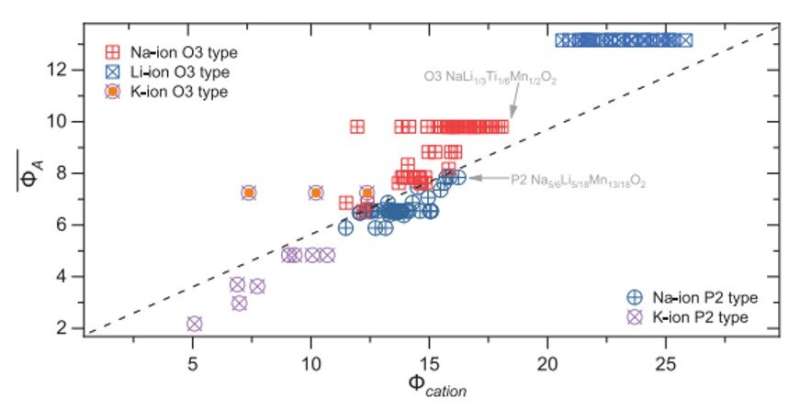
Zhao et al. therefore aimed at a simple descriptor for layered oxides and expressed the extent of the cation density and its polarizability, normalized to the ionic potential anion by defining the "cationic potential." Then they used the cationic potential as a guide to design specific stacking structures by controlling the sodium content and transition metal composition. As a starting point, they used manganese-based layered oxide cathodes (NaLi1/3Mn2/3O2), while theoretical calculations indicated the compound to be stable in an O3-type structure, the composition itself remains to be experimentally prepared in that orientation. Zhao et al. therefore lowered the cationic potential as a possible route to experimentally yield the expected O3-type structure by substituting the manganese cation (Mn4+) with a titanium cation (Ti4+) containing a lower ionic potential to form titanium-based layered oxide cathodes (NaLi1/3Ti1/6Mn1/2O2) in the expected O3-type configuration. The team confirmed the outcome using X-ray diffraction patterns to show a layered rock-salt structure, where sodium superoxide (NaO2) layers alternated with the mixed titanium-based layered oxide (Li1/3Ti1/6Mn1/2O2).
Extending the cationic potential to other metal layered oxides
The team then used the cationic potential to design a P2-type structure aimed at an anomalously high Na-content, starting as before from manganese-based layered oxide cathodes (NaLi1/3Mn2/3O2). In this instance, to prevent the formation of an O3-type structure, they increased the cationic potential by increasing the ionic potential at the transition metal sites. The resulting composition maintained a successfully prepared P2-type structure with a high sodium content and also had a higher capacity electric charge of more than 200 milliampere hours (mAh) g−1. The team extended the cationic potential to other alkali metal layered oxides, including lithium and potassium ions, where the resulting cationic potential increased from potassium to sodium to lithium. The work showed how the cationic potential could predict the structure of new sodium/transition metal oxide layered materials relative to specific compositional demands.
Outlook
In this way, Chenglong Zhao and colleagues demonstrated the ionic potential as a measure of the polarization of ions to reflect the influence of electrostatic energy on the system. The team proposed the cationic potential method to distinguish and design materials useful for sodium-ion layered oxides. The cationic potential approach developed here, however, does not apply for several conditions, including disordered compounds resulting from mechanical milling or oxides prepared under specific conditions. The cationic potential only predicted if the proposed material would crystallize in a P- or O-type structure. The team aim to gain further insights to the structural information to decide if the corresponding material is stable or practical relative to specific compositional demands in materials engineering.
More information: Zhao C. et al. Rational design of layered oxide materials for sodium-ion batteries, Science, DOI: 10.1126/science.aay9972
Bianchini M. et al. The interplay between thermodynamics and kinetics in the solid-state synthesis of layered oxides, Nature Materials, doi.org/10.1038/s41563-020-0688-6
© 2020 Science X Network















