Generating energy from salt concentration differences between sea water and river water

Generating energy from the difference in salt concentration between sea water and river water sounds like magic, yet it really works! Blue energy, as this rather obscure form of sustainable power is commonly known, has huge potential. In theory, an average river could produce as much blue energy as a hydropower plant generating power using a waterfall 142 meters in height! Ph.D. researcher Diego Pintossi has developed new ways of understanding and solving the problem of dirt clogging up the membranes used in generating blue energy. He will defend his thesis on Friday June 11th at TU/e.
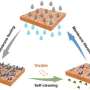
Blue energy uses a technology known as reverse electrodialysis (RED) in which the positively and negatively charged salt ions present in river and sea water move through membranes called ion exchange membranes. This movement of charge generates electrical power.
The whole process is based on the idea that sea water has lots of salt and river water very little. We can use the differences in salt concentrations between the liquids to generate electricity. Reverse electrodialysis, as the name suggests, use the same process as electrodialysis, a technology commonly used to clean or desalinate water, but in the reverse order.
Stacking up the power
The mixing of water flows containing different salt concentrations is a spontaneous process due to entropy. Think of it as nature spontaneously seeking to create a state of equilibrium," explains researcher Diego Pintossi, who is part of the Membrane Materials and Processes group of professor Kitty Nijmeijer and did his research at Wetsus, a European research center for sustainable water technology based in Leeuwarden.
"By placing membranes between the two flows, you can select which salt ions are exchanged between the solutions. In this case, we use two membranes (see image): one membrane transports negatively charged chlorine anions (Cl-) and the other transports positively-charged sodium cations (Na+). The membranes are placed in stacks, just as in fuel cells, so that the individual voltage differences can be combined to produce sufficient electricity."
Blue energy is renewable and less susceptible to daily fluctuations like solar and wind, but the cost of membranes has so far held up widespread adoption of the technology. However, in recent years, there have been some exciting developments in the field. In 2014, the world's first demo pilot (50 kW) was launched at the Afsluitdijk using water from the (salty) Waddenzee and the (freshwater) IJsselmeer.
Foiling the problem of fouling
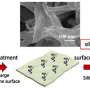
"A significant problem when using river and seawater is dirt," says Pintossi. "Components dissolved in the waters such as bacteria, clay, salts, or organic matter can accumulate on and in the membrane, thus reducing the electrical power output of the cell."
"In my research, I tried to come up with solutions for this problem, which is better known as 'fouling." First, I developed a new technique to monitor the fouling process. I found that by using electrochemical impedance spectroscopy I was able to predict at an early stage when fouling occurs. This can help in deciding when you need to clean the stacks, and how much cleaning is needed."
The researcher then looked at the influence of sulfate on the membranes. "We know that large, negatively charged particles, such as sulfate, can severely reduce the power output of the cells because they interact strongly with the membrane charges. This blocks the transport of the saline particles and affects the production of electricity."
Pintossi also developed two models to describe the effects of fouling on power generation: "These models are especially useful for predicting power generation in large scale installations. And they could help to reduce the cost of these installations."

A coating of zwitterions
Of course, identifying the cause of the fouling problem is not going to solve it. The researcher therefore also developed two successful chemical approaches that modify the membrane surface, to make it more resistant to fouling. Both use a special coating based on so-called zwitterions.
"Zwitterions, or inner salts, are molecules that contain an equal number of positively- and negatively-charged particles. Because of this, they tend to make the membranes in the RED cell more hydrophilic; in other words, they are more attracted to water. This not only delays the beginning of the onset of fouling, it also delays its growth," explains Pintossi.
"In summary, my research is an important step towards the large scale implementation of blue energy as sustainable, renewable energy source."
Machine learning
For the future, the young researcher from Italy sees much promise in the use of machine learning. "The relationship between membranes, water and fouling is extremely complex, and therefore very difficult to model in a mechanistic way."
"However, by using large amounts of data, for instance from the pilot installation at the Afsluitdijk, we will be able to build a machine learning model that relates the RED stack and water properties to the degree of RED stack fouling. This way, we can understand the process of fouling even better."
More information: Fouling in Reverse Electrodialysis: Monitoring, Modeling, and Control. pure.tue.nl/ws/portalfiles/por … 0210611_Pintossi.pdf