Keeping it clean: Engineers working to make desalination membranes stay scale-free

Boiling water can leave dark mineral deposits on the inside surface of your once-shiny aluminum kettle—nothing a little vinegar and baking soda can't scrub away.
But in other contexts, these unsightly mineral stains aren't just a nuisance—they're a major hindrance to our ability to make billions more gallons of water available to billions of people.
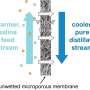
Water desalination, a technology that extracts clean water from salty, dirty sources like seawater, inland brackish water, or wastewater, is growing in popularity around the globe, with over 18,000 plants in operation worldwide. It employs a physical principle called reverse osmosis, in which dirty water is pushed at very high pressures through a membrane with pores too small to see—many times smaller than the width of a hair. Clean water is separated from a salty byproduct, and the process starts over again.
The same mineral scales that form on your kettle also form in large quantities on these specialized membranes, clogging the membranes' pores and greatly decreasing the efficiency of the entire process. Membranes must be changed or cleaned frequently, increasing costs and hindering the ability for desalination technologies to play a leading role in combating water scarcity challenges across the world.
Mineral scaling on membrane surfaces in desalination applications is a highly technical problem that Tiezheng Tong, assistant professor in Colorado State University's Department of Civil and Environmental Engineering, has spent the last several years trying to solve.
He and his students, toiling in a laboratory inside the Scott Bioengineering Building, are performing experiments and uncovering fundamental insights into why such mineral scaling happens during desalination, and what mechanisms drive the formation of scales in different types of minerals. And as engineers focus on solutions, they're also busy developing new ways to tackle the problem, whether by inventing different membranes or testing new additive solutions that would make the membranes more resistant to scaling without sacrificing water quality.
Building water resiliency
"We want our water infrastructure to be resilient to climate change," said Tong, who joined the CSU faculty in 2017 after a postdoctoral position at Yale University under Menachem Elimelech, a preeminent scholar in the field of environmental engineering. "If we run out of surface water, we need a backup to supply enough water to society … if we want to achieve this, we face a lot of challenges, including this issue of mineral scaling. It's kind of our niche, where we are working on these extremely high-salinity brine treatment processes. It's probably one of the most challenging parts of extracting water from unconventional sources."
Tong is providing his lab's expertise in desalination and membrane science to the National Alliance for Water Innovation (NAWI), a $110 million Department of Energy-led effort bringing together hundreds of scientists to tackle various problems associated with current desalination technologies. Their goal is to make desalination technologies comparable with or even cheaper than extracting fresh water from drought-stricken conventional sources like lakes, rivers and aquifers.
Through the NAWI partnership, in which CSU researchers have played a leading role, Tong's lab was recently funded alongside Vanderbilt University researchers to support the development of an electrodialysis-based method of treating hypersaline desalination brines.
The challenges of making desalination a viable choice for municipalities and countries are multi-layered: economic, in terms of building and maintaining large-scale reverse osmosis desalination plants, and environmental, in that high-salinity brines left over from water treatment processes introduces significant and damaging pollution to aquatic ecosystems.
Tong believes that solving the very specific problem of mineral scaling could have a positive ripple effect that lowers the overall cost of desalination, allowing more plants to come online, bringing new energy to bear on solving related problems like the environmental effects of hazardous brine byproducts.
The mineral scaling problem is an engineering one, but it also involves pure chemistry, with the need to understand exactly which inorganic molecules are formed on surfaces, and why. In this way, the subject appeals to Tong's multi-faceted laboratory.
"With membrane scaling, there are a lot of fundamental issues to understand," Tong said. "There are chemical reactions, kinetics and thermodynamics. In a university setting, we try to push the boundaries of the science—which is part of the excitement around working in this context. We have a lot of things to solve before we can seek answers to the real problems facing desalination technologies."
Three strategies
Tong runs a lab that focuses on three strategies for improving membrane-based water desalination. First, the engineers are trying to modify the surfaces of industrial membranes to trigger different chemistry and make them more resilient to mineral scaling.
Second, they're looking at using special chemical agents called anti-scalants that could be added to untreated water in small amounts and would hinder the formation of the scales.
The third is process innovation, or the idea of rethinking or improving entire industrial systems responsible for state-of-the-art membrane desalination.
As of late, the Tong lab has been paying increased attention to thrust 2, or the idea of perfecting anti-scalants for protecting the membranes that produce clean water. One of their most recent insights, published in Environmental Science and Technology, outlined the different behaviors of two major scalants, gypsum and silica, at molecular levels. Gypsum is formed through a crystallization process, whereas silica is formed by polymerization. These distinct mechanisms will require two types of anti-scalants with different chemical functional groups, whether negatively or positively charged.
Fourth-year Ph.D. student Yiming Yin, who has led the anti-scalant studies, said elucidating the differences between how these minerals form on membrane surfaces points to the complexity of achieving a solution for the treatment of variously salty or brackish water.
"At the moment, we have achieved a fundamental understanding of how gypsum and silica behave," said Yin. "Now we want to move on and say, what kinds of strategies can we use to mitigate them?"
Tong's students have also been capitalizing on their newfound knowledge about the chemical behavior of gypsum in particular. Using gypsum as a representative scalant, they have been experimenting with combining the techniques of reverse osmosis and membrane distillation, another type of desalination technology that applies heat to achieve pure water recovery even in the presence of high concentrations of gypsum.
While reverse osmosis is industry standard practice and is the most well-established desalination technology, it has hard limits in terms of the salinity of water it can treat. In other words, water that's a little bit dirty and saline is no problem, but once those salty, brackish concentrates get up to 70,000 milligrams per liter of salt, it becomes too difficult to push that water through the membranes, and the system breaks down. That's when membrane distillation is able to come in to finish the job, and that's why Tong's team thinks a treatment train that combines both technologies might hold the key to bringing more plants online and lowering costs. By applying anti-scalants, Tong's team has recently achieved exceptional water recovery from gypsum-containing saline waters, with their findings published in the Journal of Membrane Science.
Ronny Minjarez, a third-year undergraduate working in the lab who has co-authored the two publications on the de-scaling issue, said he was excited by the opportunity to work on technologies that have such important implications for the planet's future.
"Desalination is going to have a lot of relevance for the future, with water sources getting more and more strained," Minjarez said. "So these technologies will become even more important."
More information: Yiming Yin et al, Contrasting Behaviors between Gypsum and Silica Scaling in the Presence of Antiscalants during Membrane Distillation, Environmental Science & Technology (2021). DOI: 10.1021/acs.est.0c07190
Yiming Yin et al, The use of anti-scalants in gypsum scaling mitigation: Comparison with membrane surface modification and efficiency in combined reverse osmosis and membrane distillation, Journal of Membrane Science (2021). DOI: 10.1016/j.memsci.2021.120077