February 21, 2022 report
Using ethylene glycol to overcome issues in batteries that use zinc instead of lithium-ion
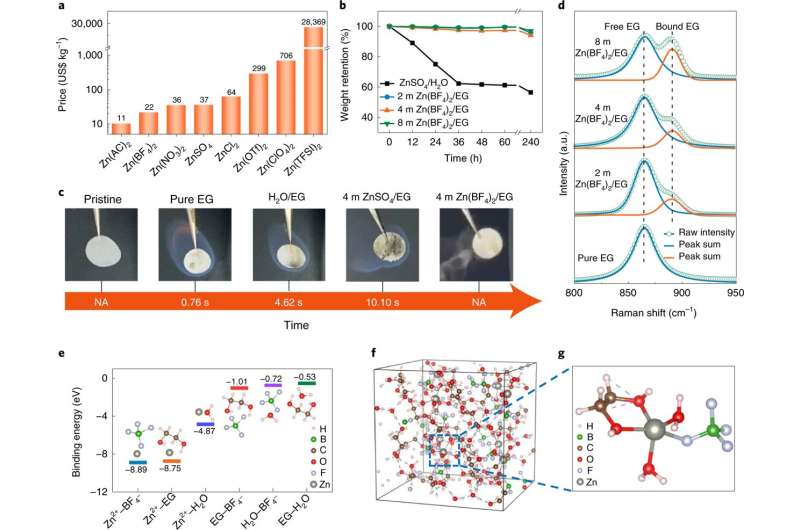
A team of researchers affiliated with several institutions in China has found a way to make batteries using zinc instead of lithium ions but without the issues traditionally associated with zinc. In their paper published in the journal Nature Sustainability, the group describes the batteries and their performance. Florencio Santos and Antonio Fernández Romero with Universidad Politécnica de Cartagena have published a News & Views piece in the same journal issue outlining general issues with using zinc to make batteries and the work done by the team in China.
As Santos and Fernández Romero note, lithium-ion batteries are dominant in portable devices and also in electric cars despite the hazards they pose during their production, use and disposal. For that reason, scientists have been looking for a suitable replacement. One such candidate is zinc—it is less toxic, is less expensive to use and can be more easily recycled. It is also widely available. Unfortunately, its use in batteries is limited by its tendency to form dendrites on electrodes, which can lead to shorting or even ignition. In this new effort, the researchers noted that the problem with zinc arises when it comes into contact with water in an electrolyte. They noted also that others have tried using water-free electrolytes, but they tend to increase production costs, and they are also not good ion conductors, and in some cases, are extremely hazardous. In their new approach, the researchers tried using zinc tetrafluoroborate hydrate as the salt and ethylene glycol as the electrolyte. This resulted in the formation of a ZnF2 layer on the electrodes that in addition to preventing the formation of dendrites also inhibited side reactions.
The electrolyte they used worked well over a wide range of temperatures and was non-flammable. They also found via testing that it could withstand 4,000 hours of cycling. Their approach shows promise as a viable alternative to lithium-ion batteries, though they acknowledge that work is required to improve current density and capacity to make such batteries competitive with the current standard.
More information: Daliang Han et al, A non-flammable hydrous organic electrolyte for sustainable zinc batteries, Nature Sustainability (2021). DOI: 10.1038/s41893-021-00800-9
Florencio Santos et al, Hydration as a solution to zinc batteries, Nature Sustainability (2021). DOI: 10.1038/s41893-021-00834-z
© 2022 Science X Network


















