Asymmetric substitution of end-groups helps to achieve efficient all-small-molecule organic solar cells
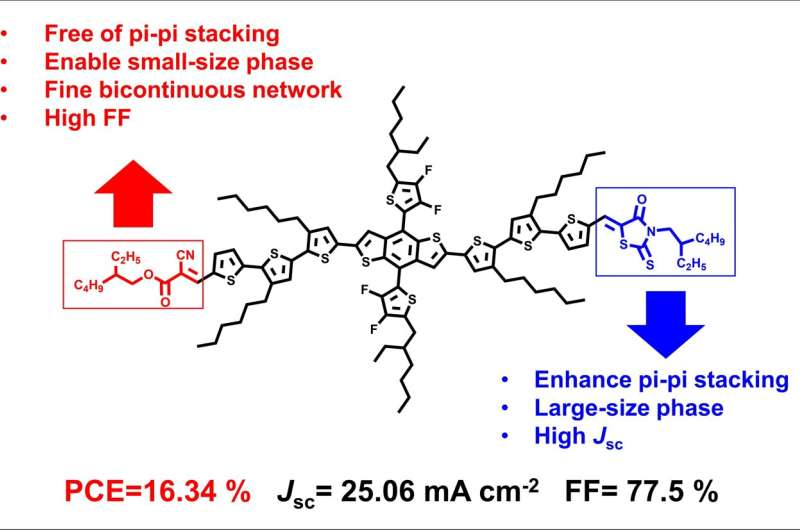
A research group led by Prof. Ge Ziyi at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences (CAS) has applied an asymmetric substitution strategy of end-groups in molecular donors, thus achieving high-performance all-small-molecule organic solar cells (OSCs) with power conversion efficiency (PCE) exceeding 16.34%. The study was published in Advanced Materials.
OSCs have attracted increasing interest due to attractive advantages such as low cost, lightweight and mechanical flexibility. As a promising power source for flexible electronic systems, in addition to relatively low PCE performance, all-small-molecule OSCs have definite chemical structures and excellent batch-to-batch reproducibility.
To break this bottleneck, the researchers designed and synthesized a series of symmetric and asymmetric small-molecule donors. The difluorothiophene substituted benzodithiophene served as a donor moiety, while 2-ethylhexyl cyanoacetate (CA), 2-ethylhexyl rhodanine (Reh) and 1H-indene-1,3(2H)-dione (ID), were selected as end-groups.
Blending with acceptor N3, SM-CA-Reh was substituted with asymmetric end-groups of CA and Reh, resulting in integrated photovoltaic advantages of high fill factor (FF) of SM-CA and high short-circuit current density (Jsc) of SM-Reh.
In this case, the device based on the SM-CA-Reh achieved an increased FF of 77.5% and a highly improved PCE of 16.34%, which was the highest PCE reported for binary all-small-molecule OSCs. By contrast, the devices based on the SM-CA-ID and SM-ID showed relatively low PCE of 8.2% and 2.76%, respectively.
In addition, characterization results indicate that the stacking morphology in blend films is mainly determined by the π–π interaction instead of dipole effect or crystallinity.
This research has revealed that the asymmetric substitution of end-groups in molecular donors can be an efficient method to further improve the all-small-molecule OSCs and illuminated how the end-groups effectively modulate the phase-separated morphology.
More information: Jinfeng Ge et al, Asymmetric Substitution of End‐Groups Triggers 16.34% Efficiency for All‐Small‐Molecule Organic Solar Cells, Advanced Materials (2022). DOI: 10.1002/adma.202202752

















